Cellular and 3D optical coherence tomography assessment during the initiation and progression of retinal degeneration in the Ccl2/Cx3cr1-deficient mouse
- PMID: 21854772
- PMCID: PMC3221782
- DOI: 10.1016/j.exer.2011.07.017
Cellular and 3D optical coherence tomography assessment during the initiation and progression of retinal degeneration in the Ccl2/Cx3cr1-deficient mouse
Abstract
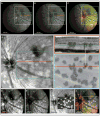
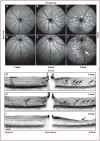
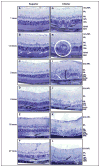
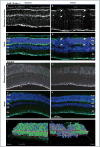
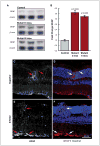
Retinal pathologies common to human eye diseases, including abnormal retinal pigment epithelial (RPE) cells, drusen-like accumulation, photoreceptor atrophy, and choroidal neovascularization, have been reported in the Ccl2/Cx3cr1-deficient mouse. The Ccl2 gene encodes the pro-inflammatory chemokine CCL2 (MCP-1), which is responsible for chemotactic recruitment of monocyte-derived macrophages to sites of inflammation. The Cx3cr1 gene encodes the fractalkine receptor, CX3CR1, and is required for accumulation of monocytes and microglia recruited via CCL2. Chemokine-mediated inflammation is implicated in retinal degenerative diseases such as diabetic retinopathy, age-related macular degeneration, retinitis pigmentosa, and uveoretinitis, and proper chemokine signaling from the RPE, Müller glia, and astrocytes is necessary to regulate leukocyte trafficking. Therefore, this mouse, possessing aberrant chemokine signaling coupled with retinal degenerative pathologies, presents an ideal opportunity to investigate the effect of altered signaling on retinal homeostasis and photoreceptor degeneration. Since this mouse is a recent development, more data covering the onset, location, and progression rate of pathologies is needed. In the present study we establish these parameters and show two photoreceptor cell death processes. Our observations of decreased glutamine synthetase and increased glial fibrillary acidic protein suggest that Müller cells respond very early within regions where lesions are forming. Finally, we suggest that retinal angiomatous proliferation contributes to pathological angiogenesis in this Ccl2/Cx3cr1-deficient mouse.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–454. - PubMed
-
- Campbell M, Humphries M, Kennan A, Kenna P, Humphries P, Brankin B. Aberrant retinal tight junction and adherens junction protein expression in an animal model of autosomal recessive Retinitis pigmentosa: The Rho(−/−) mouse. Exp Eye Res. 2006;83:484–492. - PubMed
-
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. - PubMed
-
- Carter DA, Dick AD. CD200 maintains microglial potential to migrate in adult human retinal explant model. Curr Eye Res. 2004;28:427–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

