Differential effects of mGluR7 and mGluR8 activation on pain-related synaptic activity in the amygdala
- PMID: 21854791
- PMCID: PMC3189345
- DOI: 10.1016/j.neuropharm.2011.08.006
Differential effects of mGluR7 and mGluR8 activation on pain-related synaptic activity in the amygdala
Abstract
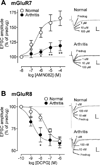
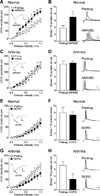
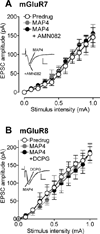
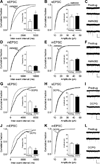
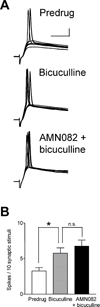
Pain-related plasticity in the laterocapsular division of the central nucleus of the amygdala (CeLC) depends on the activation of group I metabotropic glutamate receptors (mGluRs) whereas groups II and III mGluRs generally serve inhibitory functions. Recent evidence suggests differential roles of group III subtypes mGluR7 (pain enhancing) and mGluR8 (pain inhibiting) in the amygdala (Palazzo et al., 2008). Here we addressed the underlying synaptic mechanisms of mGluR7 and mGluR8 function in the CeLC under normal conditions and in an arthritis pain model. Using patch-clamp recordings in rat brain slices, we measured monosynaptic excitatory post-synaptic currents (EPSCs), mono- and polysynaptic inhibitory synaptic currents (IPSCs), and synaptically evoked action potentials (E-S coupling) in CeLC neurons. Synaptic responses were evoked by electrical stimulation in the basolateral amygdala (BLA). A selective mGluR8 agonist (DCPG) inhibited evoked EPSCs and synaptic spiking more potently in slices from arthritic rats than in slices from normal rats. In contrast, a selective mGluR7 agonist (AMN082) increased EPSCs and E-S coupling in slices from normal rats but not in the pain model. The effects of AMN082 and DCPG were blocked by a group III antagonist (MAP4). AMN082 increased frequency, but not amplitude, of spontaneous EPSCs but had no effect on miniature EPSCs (in TTX). DCPG decreased frequency, but not amplitude, of spontaneous and miniature EPSCs. The data suggest that mGluR8 acts presynaptically to inhibit excitatory transmission whereas the facilitatory effects of mGluR7 are indirect through action potential-dependent network action. AMN082 decreased evoked IPSCs and frequency, but not amplitude, of spontaneous and miniature IPSCs in slices from normal rats. DCPG had no effect on inhibitory transmission. The results suggest that presynaptic mGluR7 inhibits inhibitory synaptic transmission to gate glutamatergic transmission to CeLC neurons under normal conditions but not in pain. Presynaptic mGluR8 inhibits pain-related enhanced excitatory transmission in the CeLC.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1.Mol Pain. 2010 Dec 16;6:93. doi: 10.1186/1744-8069-6-93. Mol Pain. 2010. PMID: 21162731 Free PMC article.
-
Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors.Neuropharmacology. 2008 Sep;55(4):537-45. doi: 10.1016/j.neuropharm.2008.05.007. Epub 2008 May 16. Neuropharmacology. 2008. PMID: 18533199 Free PMC article.
-
Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala.Mol Pain. 2006 May 8;2:18. doi: 10.1186/1744-8069-2-18. Mol Pain. 2006. PMID: 16681859 Free PMC article.
-
Group II mGluRs modulate baseline and arthritis pain-related synaptic transmission in the rat medial prefrontal cortex.Neuropharmacology. 2015 Aug;95:388-94. doi: 10.1016/j.neuropharm.2015.04.003. Epub 2015 Apr 22. Neuropharmacology. 2015. PMID: 25912637 Free PMC article.
-
Angiotensin II facilitates GABAergic neurotransmission at postsynaptic sites in rat amygdala neurons.Neuropharmacology. 2018 May 1;133:334-344. doi: 10.1016/j.neuropharm.2018.02.009. Epub 2018 Feb 12. Neuropharmacology. 2018. PMID: 29447844
Cited by
-
Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females.Elife. 2018 Aug 6;7:e35656. doi: 10.7554/eLife.35656. Elife. 2018. PMID: 30079889 Free PMC article.
-
Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain.Int J Mol Sci. 2023 Jul 26;24(15):11944. doi: 10.3390/ijms241511944. Int J Mol Sci. 2023. PMID: 37569320 Free PMC article.
-
Recent Advances in the Modulation of Pain by the Metabotropic Glutamate Receptors.Cells. 2022 Aug 21;11(16):2608. doi: 10.3390/cells11162608. Cells. 2022. PMID: 36010684 Free PMC article. Review.
-
Differential effects of group III metabotropic glutamate receptors on spontaneous inhibitory synaptic currents in spine-innervating double bouquet and parvalbumin-expressing dendrite-targeting GABAergic interneurons in human neocortex.Cereb Cortex. 2023 Feb 20;33(5):2101-2142. doi: 10.1093/cercor/bhac195. Cereb Cortex. 2023. PMID: 35667019 Free PMC article.
-
Group III metabotropic glutamate receptors: pharmacology, physiology and therapeutic potential.Neurochem Res. 2014 Oct;39(10):1876-94. doi: 10.1007/s11064-014-1415-y. Epub 2014 Aug 22. Neurochem Res. 2014. PMID: 25146900 Review.
References
-
- Ansah OB, Bourbia N, Goncalves L, Almeida A, Pertovaara A. Influence of amygdaloid glutamatergic receptors on sensory and emotional pain-related behavior in the neuropathic rat. Behav. Brain Res. 2010;209:174–178. - PubMed
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

