Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain
- PMID: 21854880
- PMCID: PMC3261344
- DOI: 10.1016/j.bone.2011.08.005
Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain
Abstract
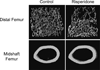
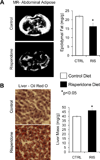
Second generation antipsychotics (SGAs) have been linked to metabolic and bone disorders in clinical studies, but the mechanisms of these side effects remain unclear. Additionally, no studies have examined whether SGAs cause bone loss in mice. Using in vivo and in vitro modeling we examined the effects of risperidone, the most commonly prescribed SGA, on bone in C57BL6/J (B6) mice. Mice were treated with risperidone orally by food supplementation at a dose of 1.25 mg/kg daily for 5 and 8 weeks, starting at 3.5 weeks of age. Risperidone reduced trabecular BV/TV, trabecular number and percent cortical area. Trabecular histomorphometry demonstrated increased resorption parameters, with no change in osteoblast number or function. Risperidone also altered adipose tissue distribution such that white adipose tissue mass was reduced and liver had significantly higher lipid infiltration. Next, in order to tightly control risperidone exposure, we administered risperidone by chronic subcutaneous infusion with osmotic minipumps (0.5 mg/kg daily for 4 weeks) in 7 week old female B6 mice. Similar trabecular and cortical bone differences were observed compared to the orally treated groups (reduced trabecular BV/TV, and connectivity density, and reduced percent cortical area) with no change in body mass, percent body fat, glucose tolerance or insulin sensitivity. Unlike in orally treated mice, risperidone infusion reduced bone formation parameters (serum P1NP, MAR and BFR/BV). Resorption parameters were elevated, but this increase did not reach statistical significance. To determine if risperidone could directly affect bone cells, primary bone marrow cells were cultured with osteoclast or osteoblast differentiation media. Risperidone was added to culture medium in clinically relevant doses of 0, 2.5 or 25 ng/ml. The number of osteoclasts was significantly increased by addition in vitro of risperidone while osteoblast differentiation was not altered. These studies indicate that risperidone treatment can have negative skeletal consequences by direct activation of osteoclast activity and by indirect non-cell autonomous mechanisms. Our findings further support the tenet that the negative side effects of SGAs on bone mass should be considered when weighing potential risks and benefits, especially in children and adolescents who have not yet reached peak bone mass.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, Lieberman JA. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. - PubMed
-
- Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32:289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

