Involvement of cytokines in slow wave sleep
- PMID: 21854954
- PMCID: PMC3645329
- DOI: 10.1016/B978-0-444-53839-0.00003-X
Involvement of cytokines in slow wave sleep
Abstract
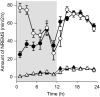
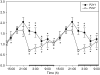
Cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL1β) play a role in sleep regulation in health and disease. TNFα or IL1β injection enhances non-rapid eye movement sleep. Inhibition of TNFα or IL1β reduces spontaneous sleep. Mice lacking TNFα or IL1β receptors sleep less. In normal humans and in multiple disease states, plasma levels of TNFα covary with EEG slow wave activity (SWA) and sleep propensity. Many of the symptoms induced by sleep loss, for example, sleepiness, fatigue, poor cognition, enhanced sensitivity to pain, are elicited by injection of exogenous TNFα or IL1β. IL1β or TNFα applied unilaterally to the surface of the cortex induces state-dependent enhancement of EEG SWA ipsilaterally, suggesting greater regional sleep intensity. Interventions such as unilateral somatosensory stimulation enhance localized sleep EEG SWA, blood flow, and somatosensory cortical expression of IL1β and TNFα. State oscillations occur within cortical columns. One such state shares properties with whole animal sleep in that it is dependent on prior cellular activity, shows homeostasis, and is induced by TNFα. Extracellular ATP released during neuro- and gliotransmission enhances cytokine release via purine type 2 receptors. An ATP agonist enhances sleep, while ATP antagonists inhibit sleep. Mice lacking the P2X7 receptor have attenuated sleep rebound responses after sleep loss. TNFα and IL1β alter neuron sensitivity by changing neuromodulator/neurotransmitter receptor expression, allowing the neuron to scale its activity to the presynaptic neurons. TNFα's role in synaptic scaling is well characterized. Because the sensitivity of the postsynaptic neuron is changed, the same input will result in a different network output signal and this is a state change. The top-down paradigm of sleep regulation requires intentional action from sleep/wake regulatory brain circuits to initiate whole-organism sleep. This raises unresolved questions as to how such purposeful action might itself be initiated. In the new paradigm, sleep is initiated within networks and local sleep is a direct consequence of prior local cell activity. Whole-organism sleep is a bottom-up, self-organizing, and emergent property of the collective states of networks throughout the brain.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Humoral sleep regulation; interleukin-1 and tumor necrosis factor.Vitam Horm. 2012;89:241-57. doi: 10.1016/B978-0-12-394623-2.00013-5. Vitam Horm. 2012. PMID: 22640617 Free PMC article. Review.
-
Unilateral cortical application of interleukin-1beta (IL1beta) induces asymmetry in fos, IL1beta and nerve growth factor immunoreactivity: implications for sleep regulation.Brain Res. 2007 Feb 2;1131(1):44-59. doi: 10.1016/j.brainres.2006.11.051. Epub 2006 Dec 20. Brain Res. 2007. PMID: 17184753
-
Biochemical regulation of sleep and sleep biomarkers.J Clin Sleep Med. 2011 Oct 15;7(5 Suppl):S38-42. doi: 10.5664/JCSM.1360. J Clin Sleep Med. 2011. PMID: 22003330 Free PMC article. Review.
-
ATP and the purine type 2 X7 receptor affect sleep.J Appl Physiol (1985). 2010 Nov;109(5):1318-27. doi: 10.1152/japplphysiol.00586.2010. Epub 2010 Sep 9. J Appl Physiol (1985). 2010. PMID: 20829501 Free PMC article.
-
Unilateral cortical application of tumor necrosis factor alpha induces asymmetry in Fos- and interleukin-1beta-immunoreactive cells within the corticothalamic projection.Brain Res. 2005 Sep 7;1055(1-2):15-24. doi: 10.1016/j.brainres.2005.06.052. Brain Res. 2005. PMID: 16098952
Cited by
-
Sleep as a translationally-relevant endpoint in studies of autism spectrum disorder (ASD).Neuropsychopharmacology. 2020 Jan;45(1):90-103. doi: 10.1038/s41386-019-0409-5. Epub 2019 May 6. Neuropsychopharmacology. 2020. PMID: 31060044 Free PMC article. Review.
-
Functions and Mechanisms of Sleep.AIMS Neurosci. 2016;3(1):67-104. doi: 10.3934/Neuroscience.2016.1.67. Epub 2016 Apr 21. AIMS Neurosci. 2016. PMID: 28413828 Free PMC article.
-
Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea.PLoS One. 2013;8(1):e54807. doi: 10.1371/journal.pone.0054807. Epub 2013 Jan 29. PLoS One. 2013. PMID: 23382975 Free PMC article. Clinical Trial.
-
Association of innate immune single-nucleotide polymorphisms with the electroencephalogram during desflurane general anaesthesia.J Mol Neurosci. 2014 Apr;52(4):497-506. doi: 10.1007/s12031-013-0201-7. Epub 2013 Dec 19. J Mol Neurosci. 2014. PMID: 24352713 Clinical Trial.
-
Sleep and Cytokines.Sleep Med Clin. 2012 Sep;7(3):517-527. doi: 10.1016/j.jsmc.2012.06.006. Sleep Med Clin. 2012. PMID: 25177229 Free PMC article. No abstract available.
References
-
- Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. The European Journal of Neuroscience. 2004;20(1):207–216. - PubMed
-
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Progress in Neurobiology. 2003;69(2):71–101. - PubMed
-
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1(3):195–204. - PubMed
-
- Burnstock G. Purinergic cotransmission. Experimental Physiology. 2009;94(1):20–24. - PubMed
-
- Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. The American Journal of Respiratory and Critical Care Medicine. 1996;153(3):1080–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

