Differential requirements for clathrin endocytic pathway components in cellular entry by Ebola and Marburg glycoprotein pseudovirions
- PMID: 21855102
- PMCID: PMC3210186
- DOI: 10.1016/j.virol.2011.07.018
Differential requirements for clathrin endocytic pathway components in cellular entry by Ebola and Marburg glycoprotein pseudovirions
Abstract
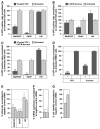
Clathrin-mediated endocytosis was previously implicated as one of the cellular pathways involved in filoviral glycoprotein mediated viral entry into target cells. Here we have further dissected the requirements for different components of this pathway in Ebola versus Marburg virus glycoprotein (GP) mediated viral infection. Although a number of these components were involved in both cases; Ebola GP-dependent viral entry specifically required the cargo recognition proteins Eps15 and DAB2 as well as the clathrin adaptor protein AP-2. In contrast, Marburg GP-mediated infection was independent of these three proteins and instead required beta-arrestin 1 (ARRB1). These findings have revealed an unexpected difference between the clathrin pathway requirements for Ebola GP versus Marburg GP pseudovirion infection. Anthrax toxin also uses a clathrin-, and ARRB1-dependent pathway for cellular entry, indicating that the mechanism used by Marburg GP pseudovirions may be more generally important for pathogen entry.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

