Polarizing agents and mechanisms for high-field dynamic nuclear polarization of frozen dielectric solids
- PMID: 21855299
- PMCID: PMC3171565
- DOI: 10.1016/j.ssnmr.2011.08.001
Polarizing agents and mechanisms for high-field dynamic nuclear polarization of frozen dielectric solids
Abstract
This article provides an overview of polarizing mechanisms involved in high-frequency dynamic nuclear polarization (DNP) of frozen biological samples at temperatures maintained using liquid nitrogen, compatible with contemporary magic-angle spinning (MAS) nuclear magnetic resonance (NMR). Typical DNP experiments require unpaired electrons that are usually exogenous in samples via paramagnetic doping with polarizing agents. Thus, the resulting nuclear polarization mechanism depends on the electron and nuclear spin interactions induced by the paramagnetic species. The Overhauser Effect (OE) DNP, which relies on time-dependent spin-spin interactions, is excluded from our discussion due the lack of conducting electrons in frozen aqueous solutions containing biological entities. DNP of particular interest to us relies primarily on time-independent, spin-spin interactions for significant electron-nucleus polarization transfer through mechanisms such as the Solid Effect (SE), the Cross Effect (CE) or Thermal Mixing (TM), involving one, two or multiple electron spins, respectively. Derived from monomeric radicals initially used in high-field DNP experiments, bi- or multiple-radical polarizing agents facilitate CE/TM to generate significant NMR signal enhancements in dielectric solids at low temperatures (<100 K). For example, large DNP enhancements (∼300 times at 5 T) from a biologically compatible biradical, 1-(TEMPO-4-oxy)-3-(TEMPO-4-amino)propan-2-ol (TOTAPOL), have enabled high-resolution MAS NMR in sample systems existing in submicron domains or embedded in larger biomolecular complexes. The scope of this review is focused on recently developed DNP polarizing agents for high-field applications and leads up to future developments per the CE DNP mechanism. Because DNP experiments are feasible with a solid-state microwave source when performed at <20K, nuclear polarization using lower microwave power (<100 mW) is possible by forcing a high proportion of biradicals to fulfill the frequency matching condition of CE (two EPR frequencies separated by the NMR frequency) using the strategies involving hetero-radical moieties and/or molecular alignment. In addition, the combination of an excited triplet and a stable radical might provide alternative DNP mechanisms without the microwave requirement.
Published by Elsevier Inc.
Figures

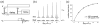


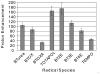

References
-
- Afeworki M, McKay RA, Schaefer J. Macromolecules. 1992;25:4084–4091.
-
- Afeworki M, Schaefer J. Macromolecules. 1992;25:4092–4096.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

