In vitro and in vivo modulation of ABCG2 by functionalized aurones and structurally related analogs
- PMID: 21855533
- PMCID: PMC3733550
- DOI: 10.1016/j.bcp.2011.08.002
In vitro and in vivo modulation of ABCG2 by functionalized aurones and structurally related analogs
Abstract
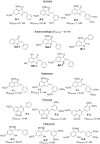
Over-expression of ABCG2 is linked to multidrug resistance in cancer chemotherapy. We have previously shown that functionalized aurones effectively reduced the efflux of pheophorbide A (an ABCG2 substrate) from ABCG2 over-expressing MDA-MB-231/R ("R") cells. In the present report, we investigated the functional relevance of this observation and the mechanisms by which it occurs. Aurones and related analogs were investigated for re-sensitization of R cells to mitoxantrone (MX, a chemotherapeutic substrate of ABCG2) in cell-based assays, accumulation of intracellular MX by cell cytometry, interaction with ABCG2 by biochemical assays and in vivo efficacy in MX resistant nude mice xenografts. We found that methoxylated aurones interacted directly with ABCG2 to inhibit efflux activity, possibly by competing for occupancy of one of the substrate binding sites on ABCG2. The present evidence suggests that they are not transported by ABCG2 although they stimulate ABCG2-ATPase activity. Alteration of ABCG2 protein expression was also discounted. One member was found to re-sensitize R cells to MX in both in vitro and in vivo settings. Our study identified methoxylated aurones as promising compounds associated with low toxicities and potent modulatory effects on the ABCG2 efflux protein. Thus, they warrant further scrutiny as lead templates for development as reversal agents of multidrug resistance.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98. - PubMed
-
- Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–76. - PubMed
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. - PubMed
-
- Boumendjel A, Florin A, Boutonnat J. Reversal agents of multidrug resistance mediated by multidrug resistance-associated proteins (MRPs) In: Boumendjel A, Boutonnat J, Robert J, editors. ABC Transporters and Multidrug Resistance. Hoboken, New Jersey: John Wiley & Sons, Inc; 2009. pp. 261–80.
-
- Lee C. Reversing agents for ATP-binding cassette drug transporters. Methods Mol Biol. 2010;596:325–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

