Angiogenin-induced tRNA fragments inhibit translation initiation
- PMID: 21855800
- PMCID: PMC3160621
- DOI: 10.1016/j.molcel.2011.06.022
Angiogenin-induced tRNA fragments inhibit translation initiation
Abstract
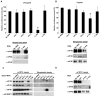
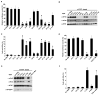
Angiogenin is a stress-activated ribonuclease that cleaves tRNA within anticodon loops to produce tRNA-derived stress-induced fragments (tiRNAs). Transfection of natural or synthetic tiRNAs inhibits protein synthesis and triggers the phospho-eIF2α-independent assembly of stress granules (SGs), essential components of the stress response program. We show that selected tiRNAs inhibit protein synthesis by displacing eIF4G/eIF4A from uncapped > capped RNAs. tiRNAs also displace eIF4F, but not eIF4E:4EBP1, from isolated m(7)G cap. We identify a terminal oligoguanine motif that is required to displace the eIF4F complex, inhibit translation, and induce SG assembly. We show that the tiRNA-associated translational silencer YB-1 contributes to angiogenin-, tiRNA-, and oxidative stress-induced translational repression. Our data reveal some of the mechanisms by which stress-induced tRNA cleavage inhibits protein synthesis and activates a cytoprotective stress response program.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
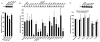
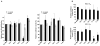

Comment in
-
Functional expansion of the tRNA world under stress.Mol Cell. 2011 Aug 19;43(4):500-2. doi: 10.1016/j.molcel.2011.08.004. Mol Cell. 2011. PMID: 21855789 Free PMC article.
References
-
- Bochkov YA, Palmenberg AC. Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. BioTechniques. 2006;41:283–284. 286, 288. passim. - PubMed
-
- Evdokimova V, Ovchinnikov LP, Sorensen PH. Y-box binding protein 1: providing a new angle on translational regulation. Cell cycle (Georgetown, Tex. 2006;5:1143–1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

