A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides
- PMID: 21855919
- PMCID: PMC3887035
- DOI: 10.1016/j.juro.2011.03.099
A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides
Erratum in
- J Urol. 2012 Feb;187(2):768
Abstract
Purpose: Studies show that LL-37 is a naturally occurring urinary defensin peptide that is up-regulated during urinary tract infections. Although normal urinary LL-37 levels are antimicrobial, we propose that increased LL-37 may trigger bladder inflammation. We further suggest that anti-inflammatory sulfated polysaccharides known as semi-synthetic glycosaminoglycan ether compounds can treat/prevent LL-37 mediated bladder inflammation.
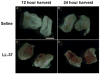
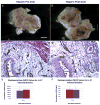
Materials and methods: C57BL/6 mice were catheterized/instilled with LL-37 (320 μM, 150 μl) for 45 minutes. Animals were sacrificed at 12 and 24 hours, and tissues were examined using hematoxylin and eosin. Separate experiments were performed for myeloperoxidase to quantify inflammation. GM-1111 semi-synthetic glycosaminoglycan ether treatments involved instillation of 10 mg/ml for 45 minutes directly before or after LL-37. Tissues were harvested at 24 hours. To compare semi-synthetic glycosaminoglycan ether efficacy, experiments were performed using 10 mg/ml heparin. Finally, tissue localization of semi-synthetic glycosaminoglycan ether was examined using a fluorescent GM-1111-Alexa Fluor® 633 conjugate.
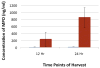
Results: Profound bladder inflammation developed after LL-37. Greater tissue inflammation occurred after 24 hours compared to that at 12 hours. Myeloperoxidase assays revealed a 21 and 61-fold increase at 12 and 24 hours, respectively. Semi-synthetic glycosaminoglycan ether treatment after LL-37 showed mild attenuation of inflammation with myeloperoxidase 2.5-fold below that of untreated bladders. Semi-synthetic glycosaminoglycan ether treatment before LL-37 demonstrated almost complete attenuation of inflammation. Myeloperoxidase results mirrored those in controls. In heparin treated bladders minimal attenuation of inflammation occurred. Finally, instillation of GM-1111-Alexa Fluor 633 revealed urothelial coating, significant tissue penetration and binding to endovasculature.
Conclusions: We developed what is to our knowledge a new model of inflammatory bladder disease by challenge with the naturally occurring urinary peptide LL-37. We also noted that a new class of anti-inflammatory sulfated polysaccharides prevents and mitigates bladder inflammation.
Copyright © 2011 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Payne CK, Joyce GF, Wise M, et al. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177:2042. - PubMed
-
- Theoharides TC, Sant GR. New agents for the medical treatment of interstitial cystitis. Expert Opin Investig Drugs. 2001;10:521. - PubMed
-
- Theoharides TC. Treatment approaches for painful bladder syndrome/interstitial cystitis. Drugs. 2007;67:215. - PubMed
-
- Toft BR, Nordling J. Recent developments of intravesical therapy of painful bladder syndrome/interstitial cystitis: a review. Curr Opin Urol. 2006;16:268. - PubMed
-
- Lukban JC, Whitmore KE, Sant GR. Current management of interstitial cystitis. Urol Clin North Am. 2002;29:649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

