Effects of pamidronate on human alveolar osteoblasts in vitro
- PMID: 21856057
- PMCID: PMC3223542
- DOI: 10.1016/j.joms.2011.05.002
Effects of pamidronate on human alveolar osteoblasts in vitro
Abstract
Purpose: Administration of bisphosphonates has recently been associated with the development of osteonecrotic lesions of the jaw (ONJ). To elucidate the potential contributions of osteogenic cells to the development and regeneration of ONJ, we have isolated primary cells from human alveolar and long/iliac bones, and examined the effects of pamidronate on cell viability, proliferation, osteogenesis, and wound healing.
Materials and methods: Primary human osteoblasts and bone marrow stromal cells were isolated from alveolar and iliac/long bone and marrow tissue. Cellular proliferation, alkaline phosphatase activity, apoptosis (terminal deoxynucleotidyl transferase dUTP nick end labeling, caspase-3, and 4,6-diamidino-2-phenylindole dihydrochloride assays) and wound healing in an in vitro scratch assay were assessed after exposure to pamidronate at a range of clinically relevant doses.
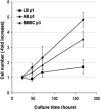
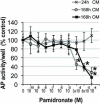
Results: Primary alveolar osteoblasts proliferated at significantly higher rates than long/iliac bone osteoblasts in vitro. Upon exposure of alveolar osteoblasts and long/iliac bone marrow stromal cells to pamidronate for more than 72 hours, we have observed significantly decreased cell viability, proliferation, osteogenesis, and in vitro wound healing at ≥6 × 10(-5) mol/L pamidronate, with the induction of apoptosis in approximately 20% of cell population.
Conclusions: The remodeling activity of alveolar bone, indicated by higher proliferation of alveolar osteoblasts, could be negatively affected by exposure to high concentrations of pamidronate over extended periods. The absence of anabolic effects of pamidronate on alveolar osteoblasts and the induction of apoptosis in osteogenic cells could negatively affect bone balance at this site and contribute to osteonecrosis of the jaw.
Copyright © 2012 American Association of Oral and Maxillofacial Surgeons. Published by Elsevier Inc. All rights reserved.
Figures




















Similar articles
-
Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate.Oral Dis. 2008 Jul;14(5):465-71. doi: 10.1111/j.1601-0825.2007.01402.x. Oral Dis. 2008. PMID: 18938273 Free PMC article.
-
The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes--in vitro study.Clin Oral Investig. 2012 Feb;16(1):87-93. doi: 10.1007/s00784-010-0507-6. Epub 2011 Jan 12. Clin Oral Investig. 2012. PMID: 21225298
-
Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro.Oral Dis. 2011 Mar;17(2):194-9. doi: 10.1111/j.1601-0825.2010.01720.x. Epub 2010 Aug 27. Oral Dis. 2011. PMID: 20796232
-
Bisphosphonates: effects on osteoblast.Eur J Clin Pharmacol. 2012 Jul;68(7):1013-8. doi: 10.1007/s00228-012-1216-7. Epub 2012 Feb 9. Eur J Clin Pharmacol. 2012. PMID: 22318756 Review.
-
[A Novel Oral Anti-osteoporosis Drug with Osteogenesis-promoting Effects via Osteoblast Differentiation].Yakugaku Zasshi. 2019;139(1):19-25. doi: 10.1248/yakushi.18-00154-2. Yakugaku Zasshi. 2019. PMID: 30606923 Review. Japanese.
Cited by
-
An in vitro assessment of fibroblast and osteoblast response to alendronate-modified titanium and the potential for decreasing fibrous encapsulation.Tissue Eng Part A. 2013 Sep;19(17-18):1919-30. doi: 10.1089/ten.TEA.2012.0218. Epub 2013 Apr 26. Tissue Eng Part A. 2013. PMID: 23540949 Free PMC article.
-
Effects of erythropoietin on osteoblast proliferation and function.Clin Exp Med. 2014 Feb;14(1):69-76. doi: 10.1007/s10238-012-0220-7. Epub 2012 Nov 8. Clin Exp Med. 2014. PMID: 23135151
-
Effect of bisphosphonate treatment of titanium surfaces on alkaline phosphatase activity in osteoblasts: a systematic review and meta-analysis.BMC Oral Health. 2020 Apr 25;20(1):125. doi: 10.1186/s12903-020-01089-4. BMC Oral Health. 2020. PMID: 32334598 Free PMC article.
-
Periosteal microcirculatory reactions in a zoledronate-induced osteonecrosis model of the jaw in rats.Clin Oral Investig. 2015 Jul;19(6):1279-88. doi: 10.1007/s00784-014-1347-6. Epub 2014 Oct 30. Clin Oral Investig. 2015. PMID: 25352470
-
Alendronate and omeprazole in combination reduce angiogenic and growth signals from osteoblasts.Bone Rep. 2021 Jan 26;14:100750. doi: 10.1016/j.bonr.2021.100750. eCollection 2021 Jun. Bone Rep. 2021. PMID: 33553512 Free PMC article.
References
-
- Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–75. - PubMed
-
- Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67:2–12. - PubMed
-
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. - PubMed
-
- Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–72. - PubMed
-
- Patel V, McLeod NM, Rogers SN, et al. Bisphosphonate osteonecrosis of the jaw-a literature review of UK policies versus international policies on bisphosphonates, risk factors and prevention. Br J Oral Maxillofac Surg. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

