FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations
- PMID: 21856723
- PMCID: PMC3170539
- DOI: 10.1093/brain/awr201
FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations
Abstract
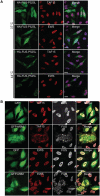
Accumulation of the DNA/RNA binding protein fused in sarcoma as cytoplasmic inclusions in neurons and glial cells is the pathological hallmark of all patients with amyotrophic lateral sclerosis with mutations in FUS as well as in several subtypes of frontotemporal lobar degeneration, which are not associated with FUS mutations. The mechanisms leading to inclusion formation and fused in sarcoma-associated neurodegeneration are only poorly understood. Because fused in sarcoma belongs to a family of proteins known as FET, which also includes Ewing's sarcoma and TATA-binding protein-associated factor 15, we investigated the potential involvement of these other FET protein family members in the pathogenesis of fused in sarcoma proteinopathies. Immunohistochemical analysis of FET proteins revealed a striking difference among the various conditions, with pathology in amyotrophic lateral sclerosis with FUS mutations being labelled exclusively for fused in sarcoma, whereas fused in sarcoma-positive inclusions in subtypes of frontotemporal lobar degeneration also consistently immunostained for TATA-binding protein-associated factor 15 and variably for Ewing's sarcoma. Immunoblot analysis of proteins extracted from post-mortem tissue of frontotemporal lobar degeneration with fused in sarcoma pathology demonstrated a relative shift of all FET proteins towards insoluble protein fractions, while genetic analysis of the TATA-binding protein-associated factor 15 and Ewing's sarcoma gene did not identify any pathogenic variants. Cell culture experiments replicated the findings of amyotrophic lateral sclerosis with FUS mutations by confirming the absence of TATA-binding protein-associated factor 15 and Ewing's sarcoma alterations upon expression of mutant fused in sarcoma. In contrast, all endogenous FET proteins were recruited into cytoplasmic stress granules upon general inhibition of Transportin-mediated nuclear import, mimicking the findings in frontotemporal lobar degeneration with fused in sarcoma pathology. These results allow a separation of fused in sarcoma proteinopathies caused by FUS mutations from those without a known genetic cause based on neuropathological features. More importantly, our data imply different pathological processes underlying inclusion formation and cell death between both conditions; the pathogenesis in amyotrophic lateral sclerosis with FUS mutations appears to be more restricted to dysfunction of fused in sarcoma, while a more global and complex dysregulation of all FET proteins is involved in the subtypes of frontotemporal lobar degeneration with fused in sarcoma pathology.
Figures






References
-
- Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2010;81:639–45. - PubMed
-
- Doi H, Koyano S, Suzuki Y, Nukina N, Kuroiwa Y. The RNA-binding protein FUS/TLS is a common aggregate-interacting protein in polyglutamine diseases. Neurosci Res. 2010;66:131–3. - PubMed
-
- Dormann D, Haass C. TDP-43 and FUS – a nuclear affair. Trends Neuroscience. 2011;34:339–48. - PubMed
-
- Groen EJ, van Es MA, van Vught PW, Spliet WG, van Engelen-Lee J, de Visser M, et al. FUS mutations in familial amyotrophic lateral sclerosis in the Netherlands. Arch Neurol. 2010;67:224–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

