Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design
- PMID: 21856766
- PMCID: PMC3756539
- DOI: 10.1158/1078-0432.CCR-11-1468
Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design
Abstract
Purpose: Treatment of patients with oncogene-addicted cancers with tyrosine kinase inhibitors (TKI) is biologically and clinically different than with cytotoxic chemotherapy. We have observed that some patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib (RECIST progression after initial benefit) have accelerated progression of disease after discontinuation of TKI. To examine this observation and define the course of patients following TKI discontinuation, we systematically evaluated patients enrolled on clinical trials of agents to treat acquired resistance to erlotinib or gefitinib.
Methods: We evaluated patients with EGFR-mutant lung cancer who participated in trials for patients with acquired resistance that mandated TKI discontinuation before administration of study therapy. Disease flare was defined as hospitalization or death attributable to disease progression during the washout period.
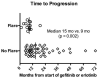
Results: Fourteen of 61 patients (23%; 95% CI: 14-35) experienced a disease flare. The median time to disease flare after TKI discontinuation was 8 days (range 3-21). Factors associated with disease flare included shorter time to progression on initial TKI (P = 0.002) and the presence of pleural (P = 0.03) or CNS disease (P = 0.01). There was no association between disease flare and the presence of T790M at the time of acquired resistance.
Conclusions: In patients with EGFR-mutant lung cancer and acquired resistance to epidermal growth factor receptor TKIs, discontinuation of erlotinib or gefitinib before initiation of study treatment is associated with a clinically significant risk of accelerated disease progression. Clinical trials in this patient population must minimize protocol-mandated washout periods.
©2011 AACR
Figures
References
-
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–46. - PubMed
-
- Kris MG, Lau CY, Ang D, Brzostowski E, Riely GJ, Rusch VW, et al. Initial results of LC-MAP: An institutional program to routinely profile tumor specimens for the presence of mutations in targetable pathways in all patients with lung adenocarcinoma. J Clin Oncol (Meeting Abstracts) 2010;28:7009.
-
- Miller VA, Johnson DH, Krug LM, Pizzo B, Tyson L, Perez W, et al. Pilot trial of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib plus carboplatin and paclitaxel in patients with stage IIIB or IV non-small-cell lung cancer. J Clin Oncol. 2003;21:2094–100. - PubMed
-
- Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–9. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous