The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii
- PMID: 21856795
- PMCID: PMC3180803
- DOI: 10.1105/tpc.111.087973
The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii
Abstract
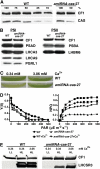
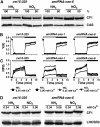
The plant-specific calcium binding protein CAS (calcium sensor) has been localized in chloroplast thylakoid membranes of vascular plants and green algae. To elucidate the function of CAS in Chlamydomonas reinhardtii, we generated and analyzed eight independent CAS knockdown C. reinhardtii lines (cas-kd). Upon transfer to high-light (HL) growth conditions, cas-kd lines were unable to properly induce the expression of LHCSR3 protein that is crucial for nonphotochemical quenching. Prolonged exposure to HL revealed a severe light sensitivity of cas-kd lines and caused diminished activity and recovery of photosystem II (PSII). Remarkably, the induction of LHCSR3, the growth of cas-kd lines under HL, and the performance of PSII were fully rescued by increasing the calcium concentration in the growth media. Moreover, perturbing cellular Ca(2+) homeostasis by application of the calmodulin antagonist W7 or the G-protein activator mastoparan impaired the induction of LHCSR3 expression in a concentration-dependent manner. Our findings demonstrate that CAS and Ca(2+) are critically involved in the regulation of the HL response and particularly in the control of LHCSR3 expression.
Figures







References
-
- Allmer J., Naumann B., Markert C., Zhang M., Hippler M. (2006). Mass spectrometric genomic data mining: Novel insights into bioenergetic pathways in Chlamydomonas reinhardtii. Proteomics 6: 6207–6220 - PubMed
-
- Berridge M.J., Irvine R.F. (1989). Inositol phosphates and cell signalling. Nature 341: 197–205 - PubMed
-
- Bouché N., Scharlat A., Snedden W., Bouchez D., Fromm H. (2002). A novel family of calmodulin-binding transcription activators in multicellular organisms. J. Biol. Chem. 277: 21851–21861 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

