Noninvasive bioluminescent imaging of primary patient acute lymphoblastic leukemia: a strategy for preclinical modeling
- PMID: 21856863
- PMCID: PMC3204743
- DOI: 10.1182/blood-2011-04-346528
Noninvasive bioluminescent imaging of primary patient acute lymphoblastic leukemia: a strategy for preclinical modeling
Abstract
The efficient engraftment in immune-deficient mice achieved with both acute lymphoblastic leukemia (ALL) cell lines and primary samples has facilitated identification of the antileukemia activity of a wide variety of agents. Despite widespread usage, however, little is known about the early ALL localization and engraftment kinetics in this model, limiting experimental read-outs primarily to survival and endpoint analysis at high disease burden. In this study, we report that bioluminescent imaging can be reproducibly achieved with primary human ALL samples. This approach provides a noninvasive, longitudinal measure of leukemia burden and localization that enhances the sensitivity of treatment response detection and provides greater insight into the mechanism of action of antileukemia agents. In addition, this study reveals significant cell line- and species-related differences in leukemia migration, especially early in expansion, which may confound observations between various leukemia models. Overall, this study demonstrates that the use of bioluminescent primary ALL allows the detection and quantitation of treatment effects at earlier, previously unquantifiable disease burdens and thus provides the means to standardize and expedite the evaluation of anti-ALL activity in preclinical xenograft studies.
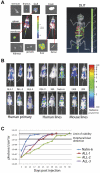
Figures


References
-
- Bernardi R, Grisendi S, Pandolfi PP. Modelling haematopoietic malignancies in the mouse and therapeutical implications. Oncogene. 2002;21(21):3445–3458. - PubMed
-
- Baersch G, Mollers T, Hotte A, et al. Good engraftment of B-cell precursor ALL in NOD-SCID mice. Klin Padiatr. 1997;209(4):178–185. - PubMed
-
- Lapidot T, Fajerman Y, Kollet O. Immune-deficient SCID and NOD/SCID mice models as functional assays for studying normal and malignant human hematopoiesis. J Mol Med. 1997;75(9):664–673. - PubMed
-
- Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. - PubMed
-
- Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

