Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability
- PMID: 21857650
- PMCID: PMC3199392
- DOI: 10.1038/emboj.2011.304
Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability
Abstract
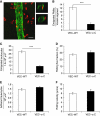
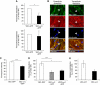
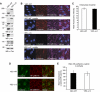
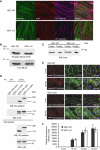
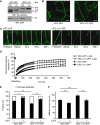
To determine whether leukocytes need to open endothelial cell contacts during extravasation, we decided to generate mice with strongly stabilized endothelial junctions. To this end, we replaced VE-cadherin genetically by a VE-cadherin-α-catenin fusion construct. Such mice were completely resistant to the induction of vascular leaks by VEGF or histamine. Neutrophil or lymphocyte recruitment into inflamed cremaster, lung and skin were strongly inhibited in these mice, documenting the importance of the junctional route in vivo. Surprisingly, lymphocyte homing into lymph nodes was not inhibited. VE-cadherin-α-catenin associated more intensely with the actin cytoskeleton as demonstrated by its membrane mobility and detergent extractability. Our results establish the junctional route as the main pathway for extravasating leukocytes in several, although not in all tissues. Furthermore, in these tissues, plasticity of the VE-cadherin-catenin complex is central for the leukocyte diapedesis mechanism.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

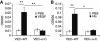
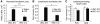

References
-
- Alksne JF (1959) The passage of colloidal particles across the dermal capillary wall under the influence of histamine. Q J Exp Physiol Cogn Med Sci 44: 51–66 - PubMed
-
- Allingham MJ, van Buul JD, Burridge K (2007) ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 179: 4053–4064 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

