Transfer RNA-mediated regulation of ribosome dynamics during protein synthesis
- PMID: 21857664
- PMCID: PMC3167956
- DOI: 10.1038/nsmb.2098
Transfer RNA-mediated regulation of ribosome dynamics during protein synthesis
Abstract
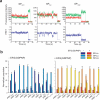
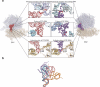
Translocation of tRNAs through the ribosome during protein synthesis involves large-scale structural rearrangement of the ribosome and ribosome-bound tRNAs that is accompanied by extensive and dynamic remodeling of tRNA-ribosome interactions. How the rearrangement of individual tRNA-ribosome interactions influences tRNA movement during translocation, however, remains largely unknown. To address this question, we used single-molecule FRET to characterize the dynamics of ribosomal pretranslocation (PRE) complex analogs carrying either wild-type or systematically mutagenized tRNAs. Our data reveal how specific tRNA-ribosome interactions regulate the rate of PRE complex rearrangement into a critical, on-pathway translocation intermediate and how these interactions control the stability of the resulting configuration. Notably, our results suggest that the conformational flexibility of the tRNA molecule has a crucial role in directing the structural dynamics of the PRE complex during translocation.
Figures




Similar articles
-
Energy barriers and driving forces in tRNA translocation through the ribosome.Nat Struct Mol Biol. 2013 Dec;20(12):1390-6. doi: 10.1038/nsmb.2690. Epub 2013 Nov 3. Nat Struct Mol Biol. 2013. PMID: 24186064
-
The ribosome as a molecular machine: the mechanism of tRNA-mRNA movement in translocation.Biochem Soc Trans. 2011 Apr;39(2):658-62. doi: 10.1042/BST0390658. Biochem Soc Trans. 2011. PMID: 21428957 Review.
-
The ribosome uses cooperative conformational changes to maximize and regulate the efficiency of translation.Proc Natl Acad Sci U S A. 2014 Aug 19;111(33):12073-8. doi: 10.1073/pnas.1401864111. Epub 2014 Aug 1. Proc Natl Acad Sci U S A. 2014. PMID: 25085895 Free PMC article.
-
Partial spontaneous intersubunit rotations in pretranslocation ribosomes.Proc Natl Acad Sci U S A. 2023 Oct 10;120(41):e2114979120. doi: 10.1073/pnas.2114979120. Epub 2023 Oct 6. Proc Natl Acad Sci U S A. 2023. PMID: 37801472 Free PMC article.
-
Structural Insights into tRNA Dynamics on the Ribosome.Int J Mol Sci. 2015 Apr 30;16(5):9866-95. doi: 10.3390/ijms16059866. Int J Mol Sci. 2015. PMID: 25941930 Free PMC article. Review.
Cited by
-
The emerging role of rectified thermal fluctuations in initiator aa-tRNA- and start codon selection during translation initiation.Biochimie. 2015 Jul;114:30-8. doi: 10.1016/j.biochi.2015.04.001. Epub 2015 Apr 14. Biochimie. 2015. PMID: 25882682 Free PMC article. Review.
-
Dynamics of ribosomes and release factors during translation termination in E. coli.Elife. 2018 Jun 11;7:e34252. doi: 10.7554/eLife.34252. Elife. 2018. PMID: 29889659 Free PMC article.
-
Comparing FRET Pairs that Report on Intersubunit Rotation in Bacterial Ribosomes.J Mol Biol. 2023 Aug 1;435(15):168185. doi: 10.1016/j.jmb.2023.168185. Epub 2023 Jun 20. J Mol Biol. 2023. PMID: 37348753 Free PMC article.
-
The UGG Isoacceptor of tRNAPro Is Naturally Prone to Frameshifts.Int J Mol Sci. 2015 Jul 1;16(7):14866-83. doi: 10.3390/ijms160714866. Int J Mol Sci. 2015. PMID: 26140378 Free PMC article.
-
Structured mRNA induces the ribosome into a hyper-rotated state.EMBO Rep. 2014 Feb;15(2):185-90. doi: 10.1002/embr.201337762. Epub 2014 Jan 8. EMBO Rep. 2014. PMID: 24401932 Free PMC article.
References
-
- Korostelev A, Noller HF. The ribosome in focus: new structures bring new insights. Trends Biochem Sci. 2007;32:434–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

