Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation
- PMID: 21857781
- PMCID: PMC3157944
- DOI: 10.2147/COPD.S10770
Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation
Abstract
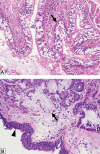
Patients with chronic obstructive pulmonary disease (COPD) exhibit dominant features of chronic bronchitis, emphysema, and/or asthma, with a common phenotype of airflow obstruction. COPD pulmonary physiology reflects the sum of pathological changes in COPD, which can occur in large central airways, small peripheral airways, and the lung parenchyma. Quantitative or high-resolution computed tomography is used as a surrogate measure for assessment of disease progression. Different biological or molecular markers have been reported that reflect the mechanistic or pathogenic triad of inflammation, proteases, and oxidants and correspond to the different aspects of COPD histopathology. Similar to the pathogenic triad markers, genetic variations or polymorphisms have also been linked to COPD-associated inflammation, protease-antiprotease imbalance, and oxidative stress. Furthermore, in recent years, there have been reports identifying aging-associated mechanistic markers as downstream consequences of the pathogenic triad in the lungs from COPD patients. For this review, the authors have limited their discussion to a review of mechanistic markers and genetic variations and their association with COPD histopathology and disease status.
Keywords: apoptosis; bronchitis; chronic obstructive pulmonary disease; emphysema; senescence.
Figures


References
-
- Wright JL, Churg A. Pathologic features of chronic obstructive pulmonary disease: diagnostic criteria and differential diagnosis. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, editors. Fishman’s pulmonary diseases and disorders. 4th ed. Vol. 1. New York: McGraw Hill; 2008. pp. 693–705.
-
- Roggli V, Cagle P. Emphysema and chronic bronchitis. In: Tomashefski J, Cagle P, Farver C, Fraire A, editors. Dail and hammar’s pulmonary pathology. 3rd ed. New York: Springer; 2008. pp. 866–885.
-
- American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–S121. - PubMed
-
- Pratt P. Emphysema and chronic airways disease. In: Dail D, Hammar S, editors. Pulmonary pathology. 2nd ed. New York: Springer-Verlag; 1994. pp. 847–865.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

