Progesterone receptor action: defining a role in breast cancer
- PMID: 21857868
- PMCID: PMC3156468
- DOI: 10.1586/eem.11.25
Progesterone receptor action: defining a role in breast cancer
Abstract
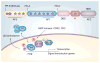
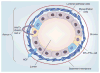
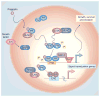
The ovarian steroid hormones, estradiol and progesterone, and their nuclear receptors (estrogen receptor [ER] and progesterone receptor [PR]), are involved in breast cancer development. As ER-positive/PR-positive tumors progress, they are likely to become steroid hormone-resistant/independent, yet often retain expression of their steroid receptors. Notably, up to 40% of women with steroid receptor-positive tumors exhibit de novo resistance or eventually fail on estrogen- or ERα-blocking therapies (acquired resistance). Indeed, most of the research on this topic has centered on mechanisms of ER 'escape' from endocrine therapy and the design of better ER-blocking strategies; signaling pathways that mediate endocrine (i.e., anti-estrogen) resistance are also excellent therapeutic targets. However, serious consideration of PR isoforms as important drivers of early breast cancer progression and ER modulators is timely and significant. Indeed, progress has been hindered by ER-centric experimental approaches. This article will focus on defining a role for PR in breast cancer with hopes of providing a refreshing PR-focused perspective.
Figures

References
-
- Tsai SY, Carlstedt-Duke J, Weigel NL, et al. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988;55(2):361–369. - PubMed
-
- Richer JK, Lange CA, Manning NG, et al. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273(47):31317–31326. - PubMed
-
- Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273(17):10696–10701. - PubMed
-
- Tseng L, Tang M, Wang Z, Mazella J. Progesterone receptor (hPR) upregulates the fibronectin promoter activity in human decidual fibroblasts. DNA Cell Biol. 2003;22(10):633–640. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
