A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204)
- PMID: 21857901
- PMCID: PMC3152265
- DOI: 10.1371/journal.pone.0021225
A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204)
Abstract
Background: The safety and immunogenicity of a vaccine regimen consisting of a 6-plasmid HIV-1 DNA prime (envA, envB, envC, gagB, polB, nefB) boosted by a recombinant adenovirus serotype-5 (rAd5) HIV-1 with matching inserts was evaluated in HIV-seronegative participants from South Africa, United States, Latin America and the Caribbean.
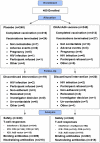
Methods: 480 participants were evenly randomized to receive either: DNA (4 mg i.m. by Biojector) at 0, 1 and 2 months, followed by rAd5 (10(10) PU i.m. by needle/syringe) at 6 months; or placebo. Participants were monitored for reactogenicity and adverse events throughout the 12-month study. Peak and duration of HIV-specific humoral and cellular immune responses were evaluated after the prime and boost.
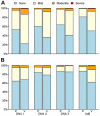
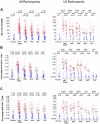
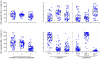
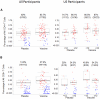
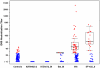
Results: The vaccine was well tolerated and safe. T-cell responses, detected by interferon-γ (IFN-γ) ELISpot to global potential T-cell epitopes (PTEs) were observed in 70.8% (136/192) of vaccine recipients overall, most frequently to Gag (54.7%) and to Env (54.2%). In U.S. vaccine recipients T-cell responses were less frequent in Ad5 sero-positive versus sero-negative vaccine recipients (62.5% versus 85.7% respectively, p = 0.035). The frequency of HIV-specific CD4+ and CD8+ T-cell responses detected by intracellular cytokine staining were similar (41.8% and 47.2% respectively) and most secreted ≥2 cytokines. The vaccine induced a high frequency (83.7%-94.6%) of binding antibody responses to consensus Group M, and Clades A, B and C gp140 Env oligomers. Antibody responses to Gag were elicited in 46% of vaccine recipients.
Conclusion: The vaccine regimen was well-tolerated and induced polyfunctional CD4+ and CD8+ T-cells and multi-clade anti-Env binding antibodies.
Trial registration: ClinicalTrials.gov NCT00125970.
Conflict of interest statement
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. 2009 AIDS epidemic update. 2009. Available: http://data.unaids.org/pub/Report/2009/jc1700_epi_update_2009_en.pdf. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. - PubMed
-
- Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, et al. Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull. 2001;58:19–42. - PubMed
-
- Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–4092. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI069420/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- U01 AI047996/AI/NIAID NIH HHS/United States
- U01 AI048023/AI/NIAID NIH HHS/United States
- U01 AI069447/AI/NIAID NIH HHS/United States
- U01 AI047985/AI/NIAID NIH HHS/United States
- UM1 AI069519/AI/NIAID NIH HHS/United States
- U01 AI069412/AI/NIAID NIH HHS/United States
- U01 AI069453/AI/NIAID NIH HHS/United States
- AI047985/AI069439/AI/NIAID NIH HHS/United States
- U01 AI069409/AI/NIAID NIH HHS/United States
- AI046747/AI068614/AI/NIAID NIH HHS/United States
- AI047980/AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069420/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069469/AI/NIAID NIH HHS/United States
- 30022/1U01AI069421/U01AI046747/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- U01 AI046725/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- AI046703/AI068635/AI/NIAID NIH HHS/United States
- AI047996/AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069409/AI/NIAID NIH HHS/United States
- U01 AI046747/AI/NIAID NIH HHS/United States
- U01 AI069469/AI/NIAID NIH HHS/United States
- U01 AI068635/AI/NIAID NIH HHS/United States
- AI069420/AI/NIAID NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- AI069519/AI/NIAID NIH HHS/United States
- U01 AI048001/AI/NIAID NIH HHS/United States
- AI048001/AI069447/AI/NIAID NIH HHS/United States
- AI048023/AI069412/AI/NIAID NIH HHS/United States
- U01 AI069519/AI/NIAID NIH HHS/United States
- AI069414/AI/NIAID NIH HHS/United States
- U01 AI047980/AI/NIAID NIH HHS/United States
- AI069453/AI/NIAID NIH HHS/United States
- U01 AI068618/AI/NIAID NIH HHS/United States
- AI046747/AI/NIAID NIH HHS/United States
- U01 AI046703/AI/NIAID NIH HHS/United States
- AI069469/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- AI069409/AI/NIAID NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- U01 AI069414/AI/NIAID NIH HHS/United States
- AI046725/AI068618/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

