miR-34a regulates mouse neural stem cell differentiation
- PMID: 21857907
- PMCID: PMC3153928
- DOI: 10.1371/journal.pone.0021396
miR-34a regulates mouse neural stem cell differentiation
Abstract
Background: MicroRNAs (miRNAs or miRs) participate in the regulation of several biological processes, including cell differentiation. Recently, miR-34a has been implicated in the differentiation of monocyte-derived dendritic cells, human erythroleukemia cells, and mouse embryonic stem cells. In addition, members of the miR-34 family have been identified as direct p53 targets. However, the function of miR-34a in the control of the differentiation program of specific neural cell types remains largely unknown. Here, we investigated the role of miR-34a in regulating mouse neural stem (NS) cell differentiation.
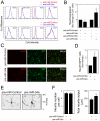
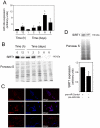
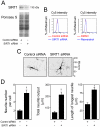
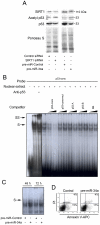
Methodology/principal findings: miR-34a overexpression increased postmitotic neurons and neurite elongation of mouse NS cells, whereas anti-miR-34a had the opposite effect. SIRT1 was identified as a target of miR-34a, which may mediate the effect of miR-34a on neurite elongation. In addition, acetylation of p53 (Lys 379) and p53-DNA binding activity were increased and cell death unchanged after miR-34a overexpression, thus reinforcing the role of p53 during neural differentiation. Interestingly, in conditions where SIRT1 was activated by pharmacologic treatment with resveratrol, miR-34a promoted astrocytic differentiation, through a SIRT1-independent mechanism.
Conclusions: Our results provide new insight into the molecular mechanisms by which miR-34a modulates neural differentiation, suggesting that miR-34a is required for proper neuronal differentiation, in part, by targeting SIRT1 and modulating p53 activity.
Conflict of interest statement
Figures

References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. - PubMed
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

