Dysregulated LRRK2 signaling in response to endoplasmic reticulum stress leads to dopaminergic neuron degeneration in C. elegans
- PMID: 21857923
- PMCID: PMC3153934
- DOI: 10.1371/journal.pone.0022354
Dysregulated LRRK2 signaling in response to endoplasmic reticulum stress leads to dopaminergic neuron degeneration in C. elegans
Abstract
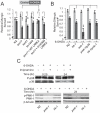
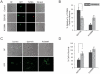
Mutation of leucine-rich repeat kinase 2 (LRRK2) is the leading genetic cause of Parkinson's Disease (PD), manifested as age-dependent dopaminergic neurodegeneration, but the underlying molecular mechanisms remain unclear. Multiple roles of LRRK2 may contribute to dopaminergic neurodegeneration. Endoplasmic reticulum (ER) stress has also been linked to PD pathogenesis, but its interactive mechanism with PD genetic factors is largely unknown. Here, we used C. elegans, human neuroblastoma cells and murine cortical neurons to determine the role of LRRK2 in maintaining dopaminergic neuron viability. We found that LRRK2 acts to protect neuroblastoma cells and C. elegans dopaminergic neurons from the toxicity of 6-hydroxydopamine and/or human α-synuclein, possibly through the p38 pathway, by supporting upregulation of GRP78, a key cell survival molecule during ER stress. A pathogenic LRRK2 mutant (G2019S), however, caused chronic p38 activation that led to death of murine neurons and age-related dopaminergic-specific neurodegeneration in nematodes. These observations establish a critical functional link between LRRK2 and ER stress.
Conflict of interest statement
Figures





Similar articles
-
6-OHDA-induced dopaminergic neurodegeneration in Caenorhabditis elegans is promoted by the engulfment pathway and inhibited by the transthyretin-related protein TTR-33.PLoS Genet. 2018 Jan 18;14(1):e1007125. doi: 10.1371/journal.pgen.1007125. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29346382 Free PMC article.
-
(G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD.Cell Death Differ. 2012 Oct;19(10):1623-33. doi: 10.1038/cdd.2012.42. Epub 2012 Apr 27. Cell Death Differ. 2012. PMID: 22539006 Free PMC article.
-
Regulation of TIR-1/SARM-1 by miR-71 Protects Dopaminergic Neurons in a C. elegans Model of LRRK2-Induced Parkinson's Disease.Int J Mol Sci. 2024 Aug 13;25(16):8795. doi: 10.3390/ijms25168795. Int J Mol Sci. 2024. PMID: 39201481 Free PMC article.
-
Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson's disease, Alzheimer's disease and autism spectrum disorder.Invert Neurosci. 2011 Dec;11(2):73-83. doi: 10.1007/s10158-011-0126-1. Epub 2011 Nov 8. Invert Neurosci. 2011. PMID: 22068627 Review.
-
Modelling the functional genomics of Parkinson's disease in Caenorhabditis elegans: LRRK2 and beyond.Biosci Rep. 2021 Sep 30;41(9):BSR20203672. doi: 10.1042/BSR20203672. Biosci Rep. 2021. PMID: 34397087 Free PMC article. Review.
Cited by
-
GRP78/BIP/HSPA5 as a Therapeutic Target in Models of Parkinson's Disease: A Mini Review.Adv Pharmacol Sci. 2019 Mar 5;2019:2706783. doi: 10.1155/2019/2706783. eCollection 2019. Adv Pharmacol Sci. 2019. PMID: 30949202 Free PMC article. Review.
-
Interaction of LRRK2 with kinase and GTPase signaling cascades.Front Mol Neurosci. 2014 Jul 9;7:64. doi: 10.3389/fnmol.2014.00064. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25071441 Free PMC article. Review.
-
Endoplasmic Reticulum Stress PERK-ATF4-CHOP Pathway Is Associated with Hypothalamic Neuronal Injury in Different Durations of Stress in Rats.Front Neurosci. 2017 Mar 24;11:152. doi: 10.3389/fnins.2017.00152. eCollection 2017. Front Neurosci. 2017. PMID: 28392758 Free PMC article.
-
Epigallocatechin-3-gallate: a useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases?EPMA J. 2013 Feb 18;4(1):5. doi: 10.1186/1878-5085-4-5. EPMA J. 2013. PMID: 23418936 Free PMC article.
-
Role of Endoplasmic Reticulum Stress and Unfolded Protein Responses in Health and Diseases.Indian J Clin Biochem. 2016 Apr;31(2):127-37. doi: 10.1007/s12291-015-0502-4. Epub 2015 Jun 16. Indian J Clin Biochem. 2016. PMID: 27069320 Free PMC article. Review.
References
-
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. - PubMed
-
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. - PubMed
-
- Paisan-Ruiz C. LRRK2 gene variation and its contribution to Parkinson disease. Hum Mutat. 2009;30:1153–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

