Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy
- PMID: 21858002
- PMCID: PMC3152547
- DOI: 10.1371/journal.pone.0023094
Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy
Abstract
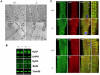
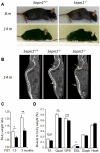
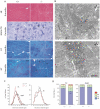
Selenium is an essential trace element and selenoprotein N (SelN) was the first selenium-containing protein shown to be directly involved in human inherited diseases. Mutations in the SEPN1 gene, encoding SelN, cause a group of muscular disorders characterized by predominant affection of axial muscles. SelN has been shown to participate in calcium and redox homeostasis, but its pathophysiological role in skeletal muscle remains largely unknown. To address SelN function in vivo, we generated a Sepn1-null mouse model by gene targeting. The Sepn1(-/-) mice had normal growth and lifespan, and were macroscopically indistinguishable from wild-type littermates. Only minor defects were observed in muscle morphology and contractile properties in SelN-deficient mice in basal conditions. However, when subjected to challenging physical exercise and stress conditions (forced swimming test), Sepn1(-/-) mice developed an obvious phenotype, characterized by limited motility and body rigidity during the swimming session, as well as a progressive curvature of the spine and predominant alteration of paravertebral muscles. This induced phenotype recapitulates the distribution of muscle involvement in patients with SEPN1-Related Myopathy, hence positioning this new animal model as a valuable tool to dissect the role of SelN in muscle function and to characterize the pathophysiological process.
Conflict of interest statement
Figures


Similar articles
-
Selenoprotein N in skeletal muscle: from diseases to function.J Mol Med (Berl). 2012 Oct;90(10):1095-107. doi: 10.1007/s00109-012-0896-x. Epub 2012 Apr 14. J Mol Med (Berl). 2012. PMID: 22527882 Review.
-
Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment.Ann Neurol. 2009 Jun;65(6):677-86. doi: 10.1002/ana.21644. Ann Neurol. 2009. PMID: 19557870
-
Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency.Hum Mol Genet. 2011 Feb 15;20(4):694-704. doi: 10.1093/hmg/ddq515. Epub 2010 Dec 2. Hum Mol Genet. 2011. PMID: 21131290
-
Selenoprotein N deficiency in mice is associated with abnormal lung development.FASEB J. 2013 Apr;27(4):1585-99. doi: 10.1096/fj.12-212688. Epub 2013 Jan 16. FASEB J. 2013. PMID: 23325319 Free PMC article.
-
Selenoprotein function and muscle disease.Biochim Biophys Acta. 2009 Nov;1790(11):1569-74. doi: 10.1016/j.bbagen.2009.03.002. Epub 2009 Mar 11. Biochim Biophys Acta. 2009. PMID: 19285112 Review.
Cited by
-
The Neurobiology of Selenium: Looking Back and to the Future.Front Neurosci. 2021 Feb 25;15:652099. doi: 10.3389/fnins.2021.652099. eCollection 2021. Front Neurosci. 2021. PMID: 33732108 Free PMC article. Review.
-
Selenoprotein N in skeletal muscle: from diseases to function.J Mol Med (Berl). 2012 Oct;90(10):1095-107. doi: 10.1007/s00109-012-0896-x. Epub 2012 Apr 14. J Mol Med (Berl). 2012. PMID: 22527882 Review.
-
Defective endoplasmic reticulum-mitochondria contacts and bioenergetics in SEPN1-related myopathy.Cell Death Differ. 2021 Jan;28(1):123-138. doi: 10.1038/s41418-020-0587-z. Epub 2020 Jul 13. Cell Death Differ. 2021. PMID: 32661288 Free PMC article.
-
Transcriptional activation of antioxidants may compensate for selenoprotein deficiencies in Amblyomma maculatum (Acari: Ixodidae) injected with selK- or selM-dsRNA.Insect Mol Biol. 2014 Aug;23(4):497-510. doi: 10.1111/imb.12098. Epub 2014 Apr 4. Insect Mol Biol. 2014. PMID: 24698418 Free PMC article.
-
Defining and identifying satellite cell-opathies within muscular dystrophies and myopathies.Exp Cell Res. 2022 Feb 1;411(1):112906. doi: 10.1016/j.yexcr.2021.112906. Epub 2021 Nov 3. Exp Cell Res. 2022. PMID: 34740639 Free PMC article.
References
-
- Rayman MP. Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta. 2009;1790:1533–1540. - PubMed
-
- Allmang C, Wurth L, Krol A. The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim Biophys Acta. 2009;1790:1415–1423. - PubMed
-
- Arner ES. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res. 2010;316:1296–1303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

