Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators
- PMID: 21858008
- PMCID: PMC3155537
- DOI: 10.1371/journal.pone.0023136
Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators
Erratum in
- PLoS One. 2012;7(1). doi:10.1371/annotation/7824df54-632a-48d6-bfbe-42d069d40c6e
Abstract
Background: Measurement of immune mediators and antimicrobial activity in female genital tract secretions may provide biomarkers predictive of risk for HIV-1 acquisition and surrogate markers of microbicide safety. However, optimal methods for sample collection do not exist. This study compared collection methods.
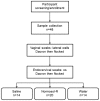
Methods: Secretions were collected from 48 women (24 with bacterial vaginosis [BV]) using vaginal and endocervical Dacron and flocked swabs. Cervicovaginal lavage (CVL) was collected with 10 mL of Normosol-R (n = 20), saline (n = 14), or water (n = 14). The concentration of gluconate in Normosol-R CVL was determined to estimate the dilution factor. Cytokine and antimicrobial mediators were measured by Luminex or ELISA and corrected for protein content. Endogenous anti-HIV-1 and anti-E. coli activity were measured by TZM-bl assay or E. coli growth.
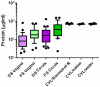
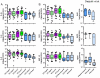
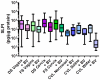
Results: Higher concentrations of protein were recovered by CVL, despite a 10-fold dilution of secretions, as compared to swab eluents. After protein correction, endocervical swabs recovered the highest mediator levels regardless of BV status. Endocervical and vaginal flocked swabs recovered significantly higher levels of anti-HIV-1 and anti-E. coli activity than Dacron swabs (P<0.001). BV had a significant effect on CVL mediator recovery. Normosol-R tended to recover higher levels of most mediators among women with BV, whereas saline or water tended to recover higher levels among women without BV. Saline recovered the highest levels of anti-HIV-1 activity regardless of BV status.
Conclusions: Endocervical swabs and CVL collected with saline provide the best recovery of most mediators and would be the optimal sampling method(s) for clinical trials.
Conflict of interest statement
Figures

References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. - PubMed
-
- Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

