Targeting RNA by small molecules: comparative structural and thermodynamic aspects of aristololactam-β-D-glucoside and daunomycin binding to tRNA(phe)
- PMID: 21858023
- PMCID: PMC3156712
- DOI: 10.1371/journal.pone.0023186
Targeting RNA by small molecules: comparative structural and thermodynamic aspects of aristololactam-β-D-glucoside and daunomycin binding to tRNA(phe)
Abstract
Background: Interaction of aristololactam-β-D-glucoside and daunomycin with tRNA(phe) was investigated using various biophysical techniques.
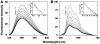
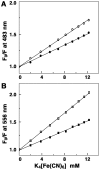
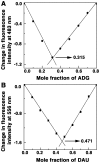
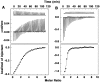
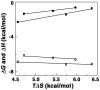
Methodology/principal findings: Absorption and fluorescence studies revealed that both the compounds bind tRNA(phe) non-cooperatively. The binding of daunomycin was about one order of magnitude higher than that of aristololactam-β-D-glucoside. Stronger binding of the former was also inferred from fluorescence quenching data, quantum efficiency values and circular dichroic results. Results from isothermal titration calorimetry experiments suggested that the binding of both compounds was predominantly entropy driven with a smaller but favorable enthalpy term that increased with temperature. A large favorable electrostatic contribution to the binding of daunomycin to tRNA(phe) was revealed from salt dependence data and the dissection of the free energy values. The electrostatic component to the free energy change for aristololactam-β-D-glucoside-tRNA(phe) interaction was smaller than that of daunomycin. This was also inferred from the slope of log K versus [Na(+)] plots. Both compounds enhanced the thermal stability of tRNA(phe). The small heat capacity changes of -47 and -99 cal/mol K, respectively, observed for aristololactam-β-D-glucoside and daunomycin, and the observed enthalpy-entropy compensation phenomenon confirmed the involvement of multiple weak noncovalent interactions. Molecular aspects of the interaction have been revealed.
Conclusions/significance: This study presents the structural and energetic aspects of the binding of aristololactam-β-D-glucoside and daunomycin to tRNA(phe).
Conflict of interest statement
Figures
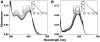
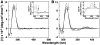

Similar articles
-
Interaction of aristololactam-β-D-glucoside and daunomycin with poly(A): spectroscopic and calorimetric studies.Biophys Chem. 2011 Apr;155(1):10-9. doi: 10.1016/j.bpc.2011.01.011. Epub 2011 Feb 16. Biophys Chem. 2011. PMID: 21392880
-
Probing the binding of two sugar bearing anticancer agents aristololactam-β-(D)-glucoside and daunomycin to double stranded RNA polynucleotides: a combined spectroscopic and calorimetric study.Mol Biosyst. 2012 Jul 6;8(7):1958-69. doi: 10.1039/c2mb25080b. Epub 2012 May 17. Mol Biosyst. 2012. PMID: 22596256
-
Binding of the plant alkaloid aristololactam-β-d-glucoside and antitumor antibiotic daunomycin to single stranded polyribonucleotides.Biochim Biophys Acta. 2013 Oct;1830(10):4708-18. doi: 10.1016/j.bbagen.2013.06.001. Epub 2013 Jun 12. Biochim Biophys Acta. 2013. PMID: 23769768
-
Natural Aristolochia Alkaloid Aristololactam-β-D-glucoside: Interaction with Biomacromolecules and Correlation to the Biological Perspectives.Mini Rev Med Chem. 2018;18(12):1022-1034. doi: 10.2174/1389557518666180222170050. Mini Rev Med Chem. 2018. PMID: 29473499 Review.
-
Molecular aspects on the interaction of protoberberine, benzophenanthridine, and aristolochia group of alkaloids with nucleic acid structures and biological perspectives.Med Res Rev. 2007 Sep;27(5):649-95. doi: 10.1002/med.20087. Med Res Rev. 2007. PMID: 16894530 Review.
Cited by
-
Insights on the comparative affinity of ribonucleic acids with plant-based beta carboline alkaloid, harmine: Spectroscopic, calorimetric and computational evaluation.Heliyon. 2024 Jul 5;10(14):e34183. doi: 10.1016/j.heliyon.2024.e34183. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39100473 Free PMC article.
-
Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA.BMC Genomics. 2013 Aug 9;14:543. doi: 10.1186/1471-2164-14-543. BMC Genomics. 2013. PMID: 23937650 Free PMC article.
-
Curcumin Regulates the r(CGG)exp RNA Hairpin Structure and Ameliorate Defects in Fragile X-Associated Tremor Ataxia Syndrome.Front Neurosci. 2020 Apr 7;14:295. doi: 10.3389/fnins.2020.00295. eCollection 2020. Front Neurosci. 2020. PMID: 32317919 Free PMC article.
-
RT-qPCR as a screening platform for mutational and small molecule impacts on structural stability of RNA tertiary structures.RSC Chem Biol. 2022 Jun 6;3(7):905-915. doi: 10.1039/d2cb00015f. eCollection 2022 Jul 6. RSC Chem Biol. 2022. PMID: 35866161 Free PMC article.
-
Antibiotic drugs targeting bacterial RNAs.Acta Pharm Sin B. 2014 Aug;4(4):258-65. doi: 10.1016/j.apsb.2014.06.012. Epub 2014 Jul 31. Acta Pharm Sin B. 2014. PMID: 26579393 Free PMC article. Review.
References
-
- Gallego J, Varani G. Targeting RNA with small-molecule drugs: Therapeutic opportunities and chemical challenges. Acc Chem Res. 2001;34:836–843. - PubMed
-
- Foloppe N, Matassova N, Aboul-Ela F. Towards the discovery of drug-like RNA ligands? Drug Discovery Today. 2006;11:1019–1027. - PubMed
-
- Fulle S, Gohlke H. Molecular recognition of RNA: Challenges for modeling interactions and plasticity. J Mol Recognit. 2010;23:220–231. - PubMed
-
- Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. The micro RNA world: Small is mighty. Trends Biochem Sci. 2003;28:534–540. - PubMed
-
- Esau CC, Monia BP. Therapeutic potential for microRNAs. Adv Drug Delivery Rev. 2007;59:101–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

