Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression
- PMID: 21858032
- PMCID: PMC3155543
- DOI: 10.1371/journal.pone.0023210
Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression
Abstract
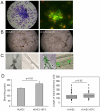
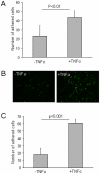
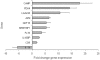
Endothelial progenitor cells (EPCs) can be purified from peripheral blood, bone marrow or cord blood and are typically defined by a limited number of cell surface markers and a few functional tests. A detailed in vitro characterization is often restricted by the low cell numbers of circulating EPCs. Therefore in vitro culturing and expansion methods are applied, which allow at least distinguishing two different types of EPCs, early and late EPCs. Herein, we describe an in vitro culture technique with the aim to generate high numbers of phenotypically, functionally and genetically defined early EPCs from human cord blood. Characterization of EPCs was done by flow cytometry, immunofluorescence microscopy, colony forming unit (CFU) assay and endothelial tube formation assay. There was an average 48-fold increase in EPC numbers. EPCs expressed VEGFR-2, CD144, CD18, and CD61, and were positive for acetylated LDL uptake and ulex lectin binding. The cells stimulated endothelial tube formation only in co-cultures with mature endothelial cells and formed CFUs. Microarray analysis revealed highly up-regulated genes, including LL-37 (CAMP), PDK4, and alpha-2-macroglobulin. In addition, genes known to be associated with cardioprotective (GDF15) or pro-angiogenic (galectin-3) properties were also significantly up-regulated after a 72 h differentiation period on fibronectin. We present a novel method that allows to generate high numbers of phenotypically, functionally and genetically characterized early EPCs. Furthermore, we identified several genes newly linked to EPC differentiation, among them LL-37 (CAMP) was the most up-regulated gene.
Conflict of interest statement
Figures




References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol. 2010;30:1088–1093. - PubMed
-
- Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

