The kinase inhibitor SFV785 dislocates dengue virus envelope protein from the replication complex and blocks virus assembly
- PMID: 21858043
- PMCID: PMC3157368
- DOI: 10.1371/journal.pone.0023246
The kinase inhibitor SFV785 dislocates dengue virus envelope protein from the replication complex and blocks virus assembly
Abstract
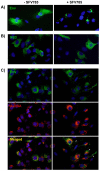
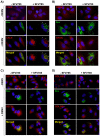
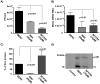
Dengue virus (DENV) is the etiologic agent for dengue fever, for which there is no approved vaccine or specific anti-viral drug. As a remedy for this, we explored the use of compounds that interfere with the action of required host factors and describe here the characterization of a kinase inhibitor (SFV785), which has selective effects on NTRK1 and MAPKAPK5 kinase activity, and anti-viral activity on Hepatitis C, DENV and yellow fever viruses. SFV785 inhibited DENV propagation without inhibiting DENV RNA synthesis or translation. The compound did not cause any changes in the cellular distribution of non-structural 3, a protein critical for DENV RNA synthesis, but altered the distribution of the structural envelope protein from a reticulate network to enlarged discrete vesicles, which altered the co-localization with the DENV replication complex. Ultrastructural electron microscopy analyses of DENV-infected SFV785-treated cells showed the presence of viral particles that were distinctly different from viable enveloped virions within enlarged ER cisternae. These viral particles were devoid of the dense nucleocapsid. The secretion of the viral particles was not inhibited by SFV785, however a reduction in the amount of secreted infectious virions, DENV RNA and capsid were observed. Collectively, these observations suggest that SFV785 inhibited the recruitment and assembly of the nucleocapsid in specific ER compartments during the DENV assembly process and hence the production of infectious DENV. SFV785 and derivative compounds could be useful biochemical probes to explore the DENV lifecycle and could also represent a new class of anti-virals.
Conflict of interest statement
Figures
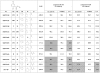
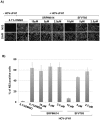
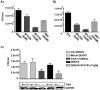
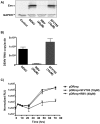

References
-
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. - PubMed
-
- Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Lippincott-Raven Publishers; 2007. pp. 1101–1152.
-
- Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect Dis. 2009;9:678–687. - PubMed
-
- Pediatric Dengue Vaccine Initiative, Global burden of dengue, International Vaccine Institute http://www.pdvi.org/about_dengue/GBD.asp 30 Mar 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

