Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana
- PMID: 21858047
- PMCID: PMC3156125
- DOI: 10.1371/journal.pone.0023255
Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana
Abstract
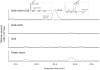
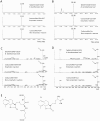
The sesquiterpene costunolide has a broad range of biological activities and is the parent compound for many other biologically active sesquiterpenes such as parthenolide. Two enzymes of the pathway leading to costunolide have been previously characterized: germacrene A synthase (GAS) and germacrene A oxidase (GAO), which together catalyse the biosynthesis of germacra-1(10),4,11(13)-trien-12-oic acid. However, the gene responsible for the last step toward costunolide has not been characterized until now. Here we show that chicory costunolide synthase (CiCOS), CYP71BL3, can catalyse the oxidation of germacra-1(10),4,11(13)-trien-12-oic acid to yield costunolide. Co-expression of feverfew GAS (TpGAS), chicory GAO (CiGAO), and chicory COS (CiCOS) in yeast resulted in the biosynthesis of costunolide. The catalytic activity of TpGAS, CiGAO and CiCOS was also verified in planta by transient expression in Nicotiana benthamiana. Mitochondrial targeting of TpGAS resulted in a significant increase in the production of germacrene A compared with the native cytosolic targeting. When the N. benthamiana leaves were co-infiltrated with TpGAS and CiGAO, germacrene A almost completely disappeared as a result of the presence of CiGAO. Transient expression of TpGAS, CiGAO and CiCOS in N. benthamiana leaves resulted in costunolide production of up to 60 ng.g(-1) FW. In addition, two new compounds were formed that were identified as costunolide-glutathione and costunolide-cysteine conjugates.
Conflict of interest statement
Figures





References
-
- Rodriguez E, Towers GHN, Mitchell JC. Biological-activies of sesquiterpene lactones. Phytochemistry. 1976;15:1573–1580.
-
- Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents. 2005;5:239–249. - PubMed
-
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone Helenalin inhibits the transcription factor NF-κB by directly targeting p65. Journal of Biological Chemistry. 1998;273:33508–33516. - PubMed
-
- Koo TH, Lee J-H, Park YJ, Hong Y-S, Kim HS, et al. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-κB by targeting IκB phosphorylation. Planta Med. 2001;67:103–107. - PubMed
-
- Klayman DL. Qinghaosu (Artemisinin) - an antimalarial drug from China. Science. 1985;228:1049–1055. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

