Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability
- PMID: 21858050
- PMCID: PMC3153490
- DOI: 10.1371/journal.pone.0023268
Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability
Abstract
Background: Dysregulation of phosphate homeostasis as occurs in chronic kidney disease is associated with cardiovascular complications. It has been suggested that both hyperphosphatemia and hypophosphatemia can cause cardiovascular disease. The molecular mechanisms by which high or low serum phosphate levels adversely affect cardiovascular function are poorly understood. The purpose of this study was to explore the mechanisms of endothelial dysfunction in the presence of non-physiologic phosphate levels.
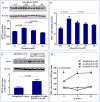
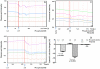
Methodology/principal findings: We studied the effects of simulated hyper- and hypophosphatemia in human umbilical vein endothelial cells in vitro. We found both simulated hyperphosphatemia and hypophosphatemia decrease eNOS expression and NO production. This was associated with reduced intracellular calcium, increased protein kinase C β2 (PKCβ2), reduced cell viability, and increased apoptosis. While simulated hyperphosphatemia was associated with decreased Akt/p-Akt, Bcl-xl/Bax ratios, NFkB-p65 and p-Erk abundance, simulated hypophosphatemia was associated with increased Akt/p-Akt and Bcl-xl/Bax ratios and p-Mek, p38, and p-p38 abundance.
Conclusions/significance: This is the first demonstration of endothelial dysfunction with hypophosphatemia. Our data suggests that both hyperphosphatemia and hypophosphatemia decrease eNOS activity via reduced intracellular calcium and increased PKCβ2. Hyperphosphatemia also appears to reduce eNOS transcription via reduced signaling through PI3K/Akt/NF-kB and MAPK/NF-kB pathways. On the other hand, hypophosphatemia appears to activate these pathways. Our data provides the basis for further studies to elucidate the relationship between altered phosphate homeostasis and cardiovascular disease. As a corollary, our data suggests that the level of phosphate in the culture media, if not in the physiologic range, may inadvertently affect experimental results.
Conflict of interest statement
Figures






References
-
- Deo R, Fyr CL, Fried LF, Newman AB, Harris TB, et al. Kidney dysfunction and fatal cardiovascular disease–an association independent of atherosclerotic events: results from the Health, Aging, and Body Composition (Health ABC) study. Am Heart J. 2008;155:62–68. - PubMed
-
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. - PubMed
-
- Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24:469–473. - PubMed
-
- Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

