Tumor-initiating cells are enriched in CD44(hi) population in murine salivary gland tumor
- PMID: 21858056
- PMCID: PMC3156741
- DOI: 10.1371/journal.pone.0023282
Tumor-initiating cells are enriched in CD44(hi) population in murine salivary gland tumor
Abstract
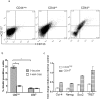
Tumor-initiating cells (T-ICs) discovered in various tumors have been widely reported. However, T-IC populations in salivary gland tumors have yet to be elucidated. Using the established Pleomorphic Adenoma Gene-1 (Plag1) transgenic mouse model of a salivary gland tumor, we identified CD44(high) (CD44(hi)) tumor cells, characterized by high levels of CD44 cell surface expression, as the T-ICs for pleomorphic adenomas. These CD44(hi) tumor cells incorporated 5-bromo-2-deoxyuridine (BrdU), at a lower rate than their CD44(negative) (CD44(neg)) counterparts, and also retained BrdU for a long period of time. Cell surface maker analysis revealed that 25% of the CD44(hi) tumor cells co-express other cancer stem cell markers such as CD133 and CD117. As few as 500 CD44(hi) tumor cells were sufficient to initiate pleomorphic adenomas in one third of the wildtype mice, whereas more than 1×10(4) CD44(neg) cells were needed for the same purpose. In NIH 3T3 cells, Plag1 was capable of activating the gene transcription of Egr1, a known upregulator for CD44. Furthermore, deletion of sequence 81-96 in the Egr1 promoter region abolished the effect of Plag1 on Egr1 upregulation. Our results establish the existence of T-ICs in murine salivary gland tumors, and suggest a potential molecular mechanism for CD44 upregulation.
Conflict of interest statement
Figures



References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

