A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2
- PMID: 21858066
- PMCID: PMC3157372
- DOI: 10.1371/journal.pone.0023310
A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2
Abstract
Background: Current human papillomavirus (HPV) vaccines that are based on virus-like particles (VLPs) of the major capsid protein L1 largely elicit HPV type-specific antibody responses. In contrast, immunization with the HPV minor capsid protein L2 elicits antibodies that are broadly cross-neutralizing, suggesting that a vaccine targeting L2 could provide more comprehensive protection against infection by diverse HPV types. However, L2-based immunogens typically elicit much lower neutralizing antibody titers than L1 VLPs. We previously showed that a conserved broadly neutralizing epitope near the N-terminus of L2 is highly immunogenic when displayed on the surface of VLPs derived from the bacteriophage PP7. Here, we report the development of a panel of PP7 VLP-based vaccines targeting L2 that protect mice from infection with carcinogenic and non-carcinogenic HPV types that infect the genital tract and skin.
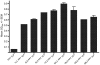
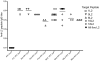
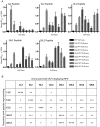
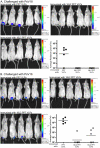
Methodology/principal findings: L2 peptides from eight different HPV types were displayed on the surface of PP7 bacteriophage VLPs. These recombinant L2 VLPs, both individually and in combination, elicited high-titer anti-L2 IgG serum antibodies. Immunized mice were protected from high dose infection with HPV pseudovirus (PsV) encapsidating a luciferase reporter. Mice immunized with 16L2 PP7 VLPs or 18L2 PP7 VLPs were nearly completely protected from both PsV16 and PsV18 challenge. Mice immunized with the mixture of eight L2 VLPs were strongly protected from genital challenge with PsVs representing eight diverse HPV types and cutaneous challenge with HPV5 PsV.
Conclusion/significance: VLP-display of a cross-neutralizing HPV L2 epitope is an effective approach for inducing high-titer protective neutralizing antibodies and is capable of offering protection from a spectrum of HPVs associated with cervical cancer as well as genital and cutaneous warts.
Conflict of interest statement
Figures




References
-
- Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of Small Virus-like Particles Assembled from the L1 Protein of Human Papillomavirus 16. Molecular Cell. 2000;5:557–567. - PubMed
-
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

