A booster vaccine expressing a latency-associated antigen augments BCG induced immunity and confers enhanced protection against tuberculosis
- PMID: 21858087
- PMCID: PMC3157374
- DOI: 10.1371/journal.pone.0023360
A booster vaccine expressing a latency-associated antigen augments BCG induced immunity and confers enhanced protection against tuberculosis
Abstract
Background: In spite of a consistent protection against tuberculosis (TB) in children, Mycobacterium bovis Bacille Calmette-Guerin (BCG) fails to provide adequate protection against the disease in adults as well as against reactivation of latent infections or exogenous reinfections. It has been speculated that failure to generate adequate memory T cell response, elicitation of inadequate immune response against latency-associated antigens and inability to impart long-term immunity against M. tuberculosis infections are some of the key factors responsible for the limited efficiency of BCG in controlling TB.
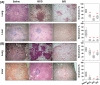
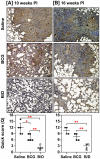
Methods/principal findings: In this study, we evaluated the ability of a DNA vaccine expressing α-crystallin--a key latency antigen of M. tuberculosis to boost the BCG induced immunity. 'BCG prime-DNA boost' regimen (B/D) confers robust protection in guinea pigs along with a reduced pathology in comparison to BCG vaccination (1.37 log(10) and 1.96 log(10) fewer bacilli in lungs and spleen, respectively; p<0.01). In addition, B/D regimen also confers enhanced protection in mice. Further, we show that B/D immunization in mice results in a heightened frequency of PPD and antigen specific multi-functional CD4 T cells (3(+)) simultaneously producing interferon (IFN)γ, tumor necrosis factor (TNF)α and interleukin (IL)2.
Conclusions/significance: These results clearly indicate the superiority of α-crystallin based B/D regimen over BCG. Our study, also demonstrates that protection against TB is predictable by an increased frequency of 3(+) Th1 cells with superior effector functions. We anticipate that this study would significantly contribute towards the development of superior booster vaccines for BCG vaccinated individuals. In addition, this regimen can also be expected to reduce the risk of developing active TB due to reactivation of latent infection.
Conflict of interest statement
Figures




References
-
- Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. - PubMed
-
- Smith PGaMAR., editor. Epidemiology of tuberculosis. Washington, D.C: American Society for Microbiology Press.; 1994. 637
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

