Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer
- PMID: 21858154
- PMCID: PMC3156234
- DOI: 10.1371/journal.pone.0023524
Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer
Abstract
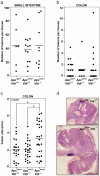
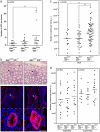
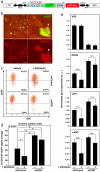
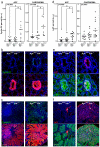
Aberrant activation of the Wnt/β-catenin pathway is critical for the initiation and progression of most colon cancers. This activation provokes the accumulation of nuclear β-catenin and the induction of its target genes. Apc(min/+) mice are the most commonly used model for colon cancer. They harbor a mutated Apc allele and develop intestinal adenomas and carcinomas during the first months of life. This phenotype is caused by the mutation of the second Apc allele and the consequent accumulation of nuclear β-catenin in the affected cells. Here we describe that vitamin D receptor (VDR) is a crucial modulator of nuclear β-catenin levels in colon cancer in vivo. By appropriate breeding of Apc(min/+) mice and Vdr(+/-) mice we have generated animals expressing a mutated Apc allele and two, one, or none Vdr wild type alleles. Lack of Vdr increased the number of colonic Aberrant Crypt Foci (ACF) but not that of adenomas or carcinomas in either small intestine or colon. Importantly, colon ACF and tumors of Apc(min/+)Vdr(-/-) mice had increased nuclear β-catenin and the tumors reached a larger size than those of Apc(min/+)Vdr(+/+). Both ACF and carcinomas in Apc(min/+)Vdr(-/-) mice showed higher expression of β-catenin/TCF target genes. In line with this, VDR knock-down in cultured human colon cancer cells enhanced β-catenin nuclear content and target gene expression. Consistently, VDR depletion abrogated the capacity of 1,25(OH)(2)D(3) to promote the relocation of β-catenin from the nucleus to the plasma membrane and to inhibit β-catenin/TCF target genes. In conclusion, VDR controls the level of nuclear β-catenin in colon cancer cells and can therefore attenuate the impact of oncogenic mutations that activate the Wnt/β-catenin pathway.
Conflict of interest statement
Figures

References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. - PubMed
-
- Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. - PubMed
-
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. - PubMed
-
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

