Probing the interaction of the diarylquinoline TMC207 with its target mycobacterial ATP synthase
- PMID: 21858172
- PMCID: PMC3157398
- DOI: 10.1371/journal.pone.0023575
Probing the interaction of the diarylquinoline TMC207 with its target mycobacterial ATP synthase
Abstract
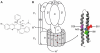
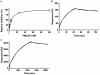
Infections with Mycobacterium tuberculosis are substantially increasing on a worldwide scale and new antibiotics are urgently needed to combat concomitantly emerging drug-resistant mycobacterial strains. The diarylquinoline TMC207 is a highly promising drug candidate for treatment of tuberculosis. This compound kills M. tuberculosis by binding to a new target, mycobacterial ATP synthase. In this study we used biochemical assays and binding studies to characterize the interaction between TMC207 and ATP synthase. We show that TMC207 acts independent of the proton motive force and does not compete with protons for a common binding site. The drug is active on mycobacterial ATP synthesis at neutral and acidic pH with no significant change in affinity between pH 5.25 and pH 7.5, indicating that the protonated form of TMC207 is the active drug entity. The interaction of TMC207 with ATP synthase can be explained by a one-site binding mechanism, the drug molecule thus binds to a defined binding site on ATP synthase. TMC207 affinity for its target decreases with increasing ionic strength, suggesting that electrostatic forces play a significant role in drug binding. Our results are consistent with previous docking studies and provide experimental support for a predicted function of TMC207 in mimicking key residues in the proton transfer chain and blocking rotary movement of subunit c during catalysis. Furthermore, the high affinity of TMC207 at low proton motive force and low pH values may in part explain the exceptional ability of this compound to efficiently kill mycobacteria in different microenvironments.
Conflict of interest statement
Figures
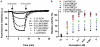


Similar articles
-
New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure.Antimicrob Agents Chemother. 2012 May;56(5):2326-34. doi: 10.1128/AAC.06154-11. Epub 2012 Feb 21. Antimicrob Agents Chemother. 2012. PMID: 22354303 Free PMC article.
-
Diarylquinolines target subunit c of mycobacterial ATP synthase.Nat Chem Biol. 2007 Jun;3(6):323-4. doi: 10.1038/nchembio884. Epub 2007 May 13. Nat Chem Biol. 2007. PMID: 17496888
-
Variations of subunit {varepsilon} of the Mycobacterium tuberculosis F1Fo ATP synthase and a novel model for mechanism of action of the tuberculosis drug TMC207.Antimicrob Agents Chemother. 2013 Jan;57(1):168-76. doi: 10.1128/AAC.01039-12. Epub 2012 Oct 22. Antimicrob Agents Chemother. 2013. PMID: 23089752 Free PMC article.
-
Unique structural and mechanistic properties of mycobacterial F-ATP synthases: Implications for drug design.Prog Biophys Mol Biol. 2020 May;152:64-73. doi: 10.1016/j.pbiomolbio.2019.11.006. Epub 2019 Nov 16. Prog Biophys Mol Biol. 2020. PMID: 31743686 Review.
-
TMC207: the first compound of a new class of potent anti-tuberculosis drugs.Future Microbiol. 2010 Jun;5(6):849-58. doi: 10.2217/fmb.10.50. Future Microbiol. 2010. PMID: 20521931 Free PMC article. Review.
Cited by
-
Bedaquiline Targets the ε Subunit of Mycobacterial F-ATP Synthase.Antimicrob Agents Chemother. 2016 Oct 21;60(11):6977-6979. doi: 10.1128/AAC.01291-16. Print 2016 Nov. Antimicrob Agents Chemother. 2016. PMID: 27620476 Free PMC article.
-
Multitarget drug discovery for tuberculosis and other infectious diseases.J Med Chem. 2014 Apr 10;57(7):3126-39. doi: 10.1021/jm500131s. Epub 2014 Apr 1. J Med Chem. 2014. PMID: 24568559 Free PMC article.
-
Structural approaches to pathway-specific antimicrobial agents.Transl Res. 2020 Jun;220:114-121. doi: 10.1016/j.trsl.2020.02.001. Epub 2020 Feb 6. Transl Res. 2020. PMID: 32105648 Free PMC article. Review.
-
Adverse Drug Reaction Monitoring in Multidrug-Resistant Tuberculosis Patients Receiving Bedaquiline and Delamanid-Based Regimen.Cureus. 2022 Oct 27;14(10):e30764. doi: 10.7759/cureus.30764. eCollection 2022 Oct. Cureus. 2022. PMID: 36447692 Free PMC article.
-
Celastrol Combats Methicillin-Resistant Staphylococcus aureus by Targeting Δ1 -Pyrroline-5-Carboxylate Dehydrogenase.Adv Sci (Weinh). 2023 Sep;10(25):e2302459. doi: 10.1002/advs.202302459. Epub 2023 Jun 28. Adv Sci (Weinh). 2023. PMID: 37381655 Free PMC article.
References
-
- Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. - PubMed
-
- Mandavilli A. Virtually incurable TB warns of impending disaster. Nat Med. 2007;13:271. - PubMed
-
- Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–43. - PubMed
-
- World Health Organization. MULTIDRUG AND EXTENSIVELY DRUG-RESISTANT TB (M/XDR-TB): 2010 GLOBAL REPORT ON SURVEILLANCE AND RESPONSE (World Health Organization 2010)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

