Implicating calpain in tau-mediated toxicity in vivo
- PMID: 21858230
- PMCID: PMC3157467
- DOI: 10.1371/journal.pone.0023865
Implicating calpain in tau-mediated toxicity in vivo
Abstract
Alzheimer's disease and other related neurodegenerative disorders known as tauopathies are characterized by the accumulation of abnormally phosphorylated and aggregated forms of the microtubule-associated protein tau. Several laboratories have identified a 17 kD proteolytic fragment of tau in degenerating neurons and in numerous cell culture models that is generated by calpain cleavage and speculated to contribute to tau toxicity. In the current study, we employed a Drosophila tauopathy model to investigate the importance of calpain-mediated tau proteolysis in contributing to tau neurotoxicity in an animal model of human neurodegenerative disease. We found that mutations that disrupted endogenous calpainA or calpainB activity in transgenic flies suppressed tau toxicity. Expression of a calpain-resistant form of tau in Drosophila revealed that mutating the putative calpain cleavage sites that produce the 17 kD fragment was sufficient to abrogate tau toxicity in vivo. Furthermore, we found significant toxicity in the fly retina associated with expression of only the 17 kD tau fragment. Collectively, our data implicate calpain-mediated proteolysis of tau as an important pathway mediating tau neurotoxicity in vivo.
Conflict of interest statement
Figures
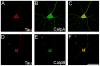
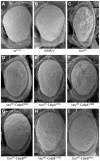
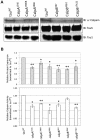
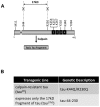
References
-
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. - PubMed
-
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. - PubMed
-
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. - PubMed
-
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

