Immunological responses in cancer patients after vaccination with the therapeutic telomerase-specific vaccine Vx-001
- PMID: 21858533
- PMCID: PMC11028568
- DOI: 10.1007/s00262-011-1093-4
Immunological responses in cancer patients after vaccination with the therapeutic telomerase-specific vaccine Vx-001
Abstract
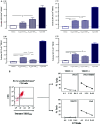
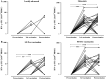
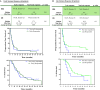
Vx-001, an HLA-A*0201 restricted telomerase (TERT)-specific anti-tumor vaccine, is composed of the 9-mer cryptic TERT(572) peptide and its optimized variant TERT(572Y). We have previously shown that Vx-001 is non-toxic, highly immunogenic and in vaccinated NSCLC patients early specific immune response is associated with prolonged survival. The aim of the present study was to investigate the specific T-cell immune response against Vx-001. Fifty-five patients with chemo-resistant advanced solid tumors were vaccinated with TERT(572Y) (2 subcutaneous injections) followed by TERT(572) peptide (4 subcutaneous injections) every 3 weeks. Specific immune response was evaluated by IFN-γ and perforin ELISpot and intracellular cytokine staining assays. TERT-reactive T cells were detected in 27 (51%) out of 53 evaluable patients after the 2nd vaccination and in 22 (69%) out of 32 evaluable patients after the completion of 6 vaccinations. Immune responses developed irrespective of the stage of disease and disease status before vaccination. Patients with disease progression at study entry who developed a post-vaccination-induced immunological response had a significant overall survival benefit compared to the post-vaccination non-responders. The Vx-001 vaccine is a promising candidate for cancer immunotherapy since it can induce a TERT-specific T-cell immune response that is associated with prolonged survival.
Conflict of interest statement
K. Kosmatopoulos and J. Menez-Jamet are employees and shareholders of Vaxon Biotech. All the other authors have no potential conflict of interest to disclose.
Figures



References
-
- Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

