Modeling of angioadaptation: insights for vascular development
- PMID: 21858766
- PMCID: PMC4940184
- DOI: 10.1387/ijdb.103218ap
Modeling of angioadaptation: insights for vascular development
Abstract
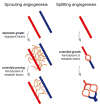
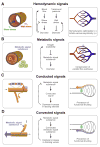
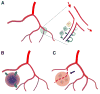
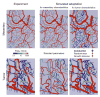
Vascular beds are generated by vasculogenesis and sprouting angiogenesis, and these processes have strong stochastic components. As a result, vascular patterns exhibit significant heterogeneity with respect to the topological arrangement of the individual vessel segments and the characteristics (length, number of segments) of different arterio-venous pathways. This structural heterogeneity tends to cause heterogeneous distributions of flow and oxygen availability in tissue. However, these quantities must be maintained within tolerable ranges to allow normal tissue function. This is achieved largely through adjustment of vascular flow resistance by control of vessel diameters. While short-term diameter control by changes in vascular tone in arterioles and small arteries plays an important role, in the long term an even more important role is played by structural adaptation (angioadaptation), occurring in response to metabolic and hemodynamic signals. The effectiveness, stability and robustness of this angioadaptation depend sensitively on the nature and strength of the vascular responses involved and their interactions with the network structure. Mathematical models are helpful in understanding these complex interactions, and can be used to simulate the consequences of failures in sensing or signal transmission mechanisms. For the tumor microcirculation, this strategy of combining experimental observations with theoretical models, has led to the hypothesis that dysfunctional information transport via vascular connexins is a major cause of the observed vascular pathology and increased heterogeneity in oxygen distribution.
Figures


Similar articles
-
Structural adaptation of normal and tumour vascular networks.Basic Clin Pharmacol Toxicol. 2012 Jan;110(1):63-9. doi: 10.1111/j.1742-7843.2011.00815.x. Epub 2011 Nov 9. Basic Clin Pharmacol Toxicol. 2012. PMID: 21995550 Free PMC article. Review.
-
Angioadaptation: keeping the vascular system in shape.News Physiol Sci. 2002 Oct;17:197-201. doi: 10.1152/nips.01395.2001. News Physiol Sci. 2002. PMID: 12270956 Review.
-
[Physiological basis of the microcirculation: vascular adaptation].Klin Monbl Augenheilkd. 2015 Feb;232(2):127-32. doi: 10.1055/s-0034-1383394. Epub 2015 Feb 20. Klin Monbl Augenheilkd. 2015. PMID: 25700251 Review. German.
-
Neural guidance molecules, tip cells, and mechanical factors in vascular development.Cardiovasc Res. 2008 May 1;78(2):232-41. doi: 10.1093/cvr/cvn058. Epub 2008 Mar 3. Cardiovasc Res. 2008. PMID: 18316324 Review.
-
Structural adaptation and heterogeneity of normal and tumor microvascular networks.PLoS Comput Biol. 2009 May;5(5):e1000394. doi: 10.1371/journal.pcbi.1000394. Epub 2009 May 29. PLoS Comput Biol. 2009. PMID: 19478883 Free PMC article.
Cited by
-
Topology and hemodynamics of the cortical cerebrovascular system.J Cereb Blood Flow Metab. 2012 Jun;32(6):952-67. doi: 10.1038/jcbfm.2012.39. Epub 2012 Apr 4. J Cereb Blood Flow Metab. 2012. PMID: 22472613 Free PMC article. Review.
-
Parallels between the Developing Vascular and Neural Systems: Signaling Pathways and Future Perspectives for Regenerative Medicine.Adv Sci (Weinh). 2021 Dec;8(23):e2101837. doi: 10.1002/advs.202101837. Epub 2021 Oct 24. Adv Sci (Weinh). 2021. PMID: 34693660 Free PMC article. Review.
-
A systems biology view of blood vessel growth and remodelling.J Cell Mol Med. 2014 Aug;18(8):1491-508. doi: 10.1111/jcmm.12164. Epub 2013 Nov 17. J Cell Mol Med. 2014. PMID: 24237862 Free PMC article. Review.
-
Efficient vasculature investment in tissues can be determined without global information.J R Soc Interface. 2020 Apr;17(165):20200137. doi: 10.1098/rsif.2020.0137. Epub 2020 Apr 22. J R Soc Interface. 2020. PMID: 32316879 Free PMC article.
-
Systems biology of the microvasculature.Integr Biol (Camb). 2015 May;7(5):498-512. doi: 10.1039/c4ib00296b. Epub 2015 Apr 2. Integr Biol (Camb). 2015. PMID: 25839068 Free PMC article. Review.
References
-
- BAKKER EN, VAN DER MEULEN ET, VAN DEN BERG BM, EVERTS V, SPAAN JA, VANBAVEL E. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J Vasc Res. 2002;39:12–20. - PubMed
-
- BURRI PH. Intussusceptive microvascular growth, a new mechanism of capillary network formation. EXS. 1992;61:32–39. - PubMed
-
- BUSCHMANN I, PRIES A, STYP-REKOWSKA B, HILLMEISTER P, LOUFRANI L, HENRION D, SHI Y, DUELSNER A, HOEFER I, GATZKE N, WANG H, LEHMANN K, ULM L, RITTER Z, HAUFF P, HLUSHCHUK R, DJONOV V, VAN VEEN T, LE NOBLE F. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–2196. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

