Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia
- PMID: 21858802
- PMCID: PMC4349201
- DOI: 10.1002/cncr.25983
Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia
Abstract
Background: Lenalidomide, an immunomodulatory agent, has activity in lymphoproliferative disorders. The authors, therefore, evaluated its effects on T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia (CLL).
Methods: To study the immunomodulatory effects of lenalidomide in CLL, the authors recruited 24 patients with untreated CLL enrolled in a phase 2 clinical trial of lenalidomide and obtained peripheral blood specimens for immunologic studies consisting of enumeration of T cells and assessing their ability to synthesize cytokines after activation through T-cell receptor (TCR).
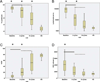
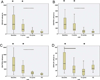
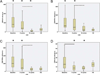
Results: After 3 cycles of therapy, patients had a significant reduction in percentage (%) and absolute lymphocyte count (ALC) and an increase in percentage of T cells, percentage of activated CD8(+) T cells producing IFN-γ, and percentage of regulatory T (T(R) ) cells when compared with their respective levels before treatment. After 15 cycles of treatment, responder patients had significant reduction in percentage of lymphocytes and ALC, percentage of activated CD4(+) T cells producing IL-2, IFN-γ, or TNF-α, and percentage of T(R) cells when compared with their perspective levels after 3 cycles of treatment. Furthermore, the numbers of activated CD4(+) T cells producing IL-2, IFN-γ, or TNF-α, activated CD8(+) T cells producing IFN-γ, and T(R) cells normalized to the range of healthy subjects.
Conclusions: Treatment with lenalidomide resulted in the normalization of functional T-cell subsets in responders, suggesting that lenalidomide may modulate cell-mediated immunity in patients with CLL.
Cancer 2011 © 2011 American Cancer Society.
Figures

Similar articles
-
Production of intracellular IL-2, TNF-alpha, and IFN-gamma by T cells in B-CLL.Cytometry B Clin Cytom. 2003 Nov;56(1):23-9. doi: 10.1002/cyto.b.10052. Cytometry B Clin Cytom. 2003. PMID: 14582134
-
Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells.Biomed Res Int. 2014;2014:265840. doi: 10.1155/2014/265840. Epub 2014 Sep 17. Biomed Res Int. 2014. PMID: 25313353 Free PMC article.
-
Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug.J Clin Invest. 2008 Jul;118(7):2427-37. doi: 10.1172/JCI35017. J Clin Invest. 2008. PMID: 18551193 Free PMC article.
-
Lenalidomide alone and in combination for chronic lymphocytic leukemia.Curr Hematol Malig Rep. 2013 Mar;8(1):7-13. doi: 10.1007/s11899-012-0146-x. Curr Hematol Malig Rep. 2013. PMID: 23254517 Review.
-
Lenalidomide and chronic lymphocytic leukemia.Biomed Res Int. 2013;2013:932010. doi: 10.1155/2013/932010. Epub 2013 Sep 19. Biomed Res Int. 2013. PMID: 24163824 Free PMC article. Review.
Cited by
-
Activation of Th1 Immunity within the Tumor Microenvironment Is Associated with Clinical Response to Lenalidomide in Chronic Lymphocytic Leukemia.J Immunol. 2018 Oct 1;201(7):1967-1974. doi: 10.4049/jimmunol.1800570. Epub 2018 Aug 13. J Immunol. 2018. PMID: 30104242 Free PMC article. Clinical Trial.
-
Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia.J Clin Oncol. 2013 Feb 10;31(5):584-91. doi: 10.1200/JCO.2012.42.8623. Epub 2012 Dec 26. J Clin Oncol. 2013. PMID: 23270003 Free PMC article. Clinical Trial.
-
Treating Multiple Myeloma in the Context of the Bone Marrow Microenvironment.Curr Oncol. 2022 Nov 21;29(11):8975-9005. doi: 10.3390/curroncol29110705. Curr Oncol. 2022. PMID: 36421358 Free PMC article. Review.
-
Safety and activity of pembrolizumab in combination with rituximab in relapsed or refractory follicular lymphoma.Blood Adv. 2022 Feb 22;6(4):1143-1151. doi: 10.1182/bloodadvances.2021006240. Blood Adv. 2022. PMID: 35015819 Free PMC article. Clinical Trial.
-
Lenalidomide-induced graft-vs.-Leukemia effect in a patient with chronic lymphocytic leukemia who relapsed after allogeneic stem cell transplant.Clin Lymphoma Myeloma Leuk. 2014 Jun;14(3):e105-9. doi: 10.1016/j.clml.2013.12.013. Epub 2013 Dec 27. Clin Lymphoma Myeloma Leuk. 2014. PMID: 24502832 Free PMC article.
References
-
- List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. http://content.nejm.org/cgi/content/abstract/352/6/549. - PubMed
-
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. http://bloodjournal.hematologylibrary.org/cgi/content/abstract/bloodjour.... - PMC - PubMed
-
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

