Toxicological and toxicokinetic analysis of angiotensin (1-7) in two species
- PMID: 21858825
- PMCID: PMC3619381
- DOI: 10.1002/jps.22730
Toxicological and toxicokinetic analysis of angiotensin (1-7) in two species
Abstract
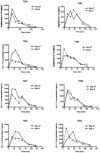
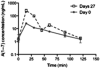
The objectives of this study were to determine the potential systemic and local toxicity, as well as evaluate the toxicokinetic (TK) profile of angiotensin (1-7) [A(1-7)] when administered daily via subcutaneous injection for 28 days to Sprague-Dawley rats and Beagle dogs. A(1-7) is a member of the renin-angiotensin system and has undergone clinical evaluation for the treatment of chemotherapy-induced myelosuppression. In this present study, A(1-7) was given at 10 mg/(kg day) for 28 days to rats and canines. At day 27, blood was harvested to evaluate the TK parameters. On day 28, systemic toxicology was evaluated. Following A(1-7) administration for 27 days, no plasma A(1-7) accumulation was detected in canines; however, increased A(1-7) plasma concentrations were detected in rats. Despite the accumulation observed in rats, no detectable toxicity was found following A(1-7) administration for 28 days. The TK analysis of A(1-7) revealed a plasma half-life of 20-30 min in both rats and canines. The time to maximum plasma concentration was found to be 15 and 26.25 min in rats and canines, respectively. This study shows that subcutaneous administration of A(1-7) at 10 mg/(kg day) for 28 days did not lead to any detectable toxicities in either rats or canines.
Copyright © 2011 Wiley-Liss, Inc.
Figures

References
-
- Lavoie JL, Sigmund CD. Minireview: Overview of the renin–angiotensin system—An endocrine and paracrine system endocrinology. Endocrinology. 2003;144(6):2179–2183. - PubMed
-
- Rodgers KE, Xiong S, diZerega GS. Effect of angiotensin II and angiotensin (1–7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol. 2003;51(2):97–106. - PubMed
-
- Rodgers KE, Xiong S, diZerega GS. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol. 2002;49(5):403–411. - PubMed
-
- Abiko M, Rodgers KE, Campeau JD, Nakamura RM, diZerega GS. Alterations of angiotensin II receptor levels in full-thickness excisional wounds in rat skin. Wound Repair Regen. 1996;4(3):363–367. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

