Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer
- PMID: 21859492
- PMCID: PMC3170584
- DOI: 10.1186/1423-0127-18-63
Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer
Abstract
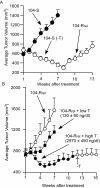
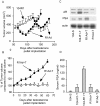
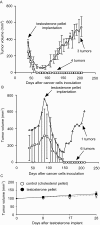
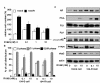
Prostate cancer is the most frequently diagnosed non-cutaneous tumor of men in Western countries. While surgery is often successful for organ-confined prostate cancer, androgen ablation therapy is the primary treatment for metastatic prostate cancer. However, this therapy is associated with several undesired side-effects, including increased risk of cardiovascular diseases. Shortening the period of androgen ablation therapy may benefit prostate cancer patients. Intermittent Androgen Deprivation therapy improves quality of life, reduces toxicity and medical costs, and delays disease progression in some patients. Cell culture and xenograft studies using androgen receptor (AR)-positive castration-resistant human prostate cancers cells (LNCaP, ARCaP, and PC-3 cells over-expressing AR) suggest that androgens may suppress the growth of AR-rich prostate cancer cells. Androgens cause growth inhibition and G1 cell cycle arrest in these cells by regulating c-Myc, Skp2, and p27Kip via AR. Higher dosages of testosterone cause greater growth inhibition of relapsed tumors. Manipulating androgen/AR signaling may therefore be a potential therapy for AR-positive advanced prostate cancer.
Figures

References
-
- Huggins C, Stevens R, Hodges C. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

