BMP-2 gene-fibronectin-apatite composite layer enhances bone formation
- PMID: 21859498
- PMCID: PMC3175450
- DOI: 10.1186/1423-0127-18-62
BMP-2 gene-fibronectin-apatite composite layer enhances bone formation
Abstract
Background: Safe and efficient gene transfer systems are needed for tissue engineering. We have developed an apatite composite layer including the bone morphogenetic protein-2 (BMP-2) gene and fibronectin (FB), and we evaluated its ability to induce bone formation.
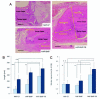
Methods: An apatite composite layer was evaluated to determine the efficiency of gene transfer to cells cultured on it. Cells were cultured on a composite layer including the BMP-2 gene and FB, and BMP-2 gene expression, BMP-2 protein concentrations, alkaline phosphatase (ALP) activity, and osteocalcin (OC) concentrations were measured. A bone defect on the cranium of rats was treated with hydroxyapatite (HAP)-coated ceramic buttons with the apatite composite layer including the BMP-2 gene and FB (HAP-BMP-FB). The tissue concentration of BMP-2, bone formation, and the expression levels of the BMP-2, ALP, and OC genes were all quantified.
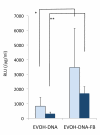
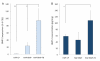
Results: The apatite composite layer provided more efficient gene transfer for the cultured cells than an apatite composite layer without FB. The BMP-2 concentration was approximately 100-600 pg/mL in the cell-culture medium. Culturing the cells on the apatite composite layer for 27 days increased ALP activity and OC concentrations. In animal experiments, the tissue concentrations of BMP-2 were over 100 pg/mg in the HAP-BMP-FB group and approximately 50 pg/mg in the control groups. Eight weeks later, bone formation was more enhanced in the HAP-BMP-FB group than in the control groups. In the tissues surrounding the HAP button, the gene expression levels of ALP and OC increased.
Conclusion: The BMP-2 gene-FB-apatite composite layer might be useful for bone engineering.
Figures



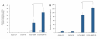
Similar articles
-
BMP-2 and ALP gene expression induced by a BMP-2 gene-fibronectin-apatite composite layer.Biomed Mater. 2011 Aug;6(4):045004. doi: 10.1088/1748-6041/6/4/045004. Epub 2011 Jun 3. Biomed Mater. 2011. PMID: 21636885
-
[A novel tissue-engineered bone constructed by using human adipose-derived stem cells and biomimetic calcium phosphate scaffold coprecipitated with bone morphogenetic protein-2].Beijing Da Xue Xue Bao Yi Xue Ban. 2017 Feb 18;49(1):6-15. Beijing Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28202997 Chinese.
-
DNA-lipid-apatite composite layers enhance gene expression of mesenchymal stem cells.Mater Sci Eng C Mater Biol Appl. 2013 Jan 1;33(1):512-8. doi: 10.1016/j.msec.2012.09.023. Epub 2012 Oct 3. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 25428103
-
Bone formation under the influence of bone morphogenetic protein/self-setting apatite cement composite as a delivery system.Biomed Mater Eng. 1994;4(4):291-307. Biomed Mater Eng. 1994. PMID: 7950877
-
Characterization of cyclic acetal hydroxyapatite nanocomposites for craniofacial tissue engineering.J Biomed Mater Res A. 2010 Aug;94(2):408-18. doi: 10.1002/jbm.a.32683. J Biomed Mater Res A. 2010. PMID: 20186741
Cited by
-
When 1+1>2: Nanostructured composites for hard tissue engineering applications.Mater Sci Eng C Mater Biol Appl. 2015 Dec 1;57:434-51. doi: 10.1016/j.msec.2015.07.050. Epub 2015 Aug 1. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 26354283 Free PMC article. Review.
-
BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry.Pharmaceutics. 2019 Aug 5;11(8):393. doi: 10.3390/pharmaceutics11080393. Pharmaceutics. 2019. PMID: 31387267 Free PMC article. Review.
-
Tauroursodeoxycholic acid combined with selenium accelerates bone regeneration in ovariectomized rats.J Mater Sci Mater Med. 2024 Oct 15;35(1):64. doi: 10.1007/s10856-024-06803-0. J Mater Sci Mater Med. 2024. PMID: 39404912 Free PMC article.
-
Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration.Int J Mol Sci. 2013 Jun 18;14(6):12714-28. doi: 10.3390/ijms140612714. Int J Mol Sci. 2013. PMID: 23778088 Free PMC article.
-
Sonoporation increases therapeutic efficacy of inducible and constitutive BMP2/7 in vivo gene delivery.Hum Gene Ther Methods. 2014 Feb;25(1):57-71. doi: 10.1089/hgtb.2013.113. Epub 2013 Nov 27. Hum Gene Ther Methods. 2014. PMID: 24164605 Free PMC article.
References
-
- Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–739. - PubMed
-
- Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Jt Surg Am. 1999;81:905–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

