Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: a GERCOR study
- PMID: 21859820
- PMCID: PMC3228179
- DOI: 10.1634/theoncologist.2011-0039
Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: a GERCOR study
Abstract
Background: The present study was done to establish a prognostic model for patients and trials using an oxaliplatin-based or irinotecan-based first-line chemotherapy in metastatic colorectal cancer.
Patients and methods: Eight hundred three patients treated with FOLFOX or FOLFIRI in three prospective trials were randomly separated into learning (n = 535) and validation (n = 268) samples. Eleven baseline variables were evaluated in univariate and multivariate analysis as prognostic factors for overall survival, and a prognostic score was developed.
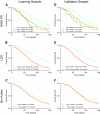
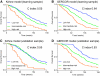
Results: Independent prognostic factors identified in multivariate analysis for overall survival were performance status (PS) (p < .001), serum lactate dehydrogenase (LDH) (p < .001), and number of metastatic sites (p = .005). A prognostic score based on these three variables was found efficient (Harrell's C index 0.61). This new model was improved by selecting only PS and LDH (Harrell's C index 0.64). Three risk groups for death could be identified: a low-risk group (n = 184; median overall survival [OS] 29.8 months), an intermediate-risk group (n = 223; median OS 19.5 months), and a high-risk group (n = 128; median OS 13.9 months). Median survival for the low-, intermediate-, and high-risk groups were 26.8, 21.1, and 16.5 months, respectively, in the validation sample (Harrell's C index 0.63).
Conclusions: Serum LDH level was the main prognostic factor in predicting survival, followed by WHO PS. We identified three risk groups for death depending on these two baseline parameters. This simple prognostic model can be useful for clinician's use and patient stratification in future clinical trials.
Conflict of interest statement
Section Editor
Section Editor
Section Editor
Reviewer “A” discloses no financial relationships.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
Figures

References
-
- Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Rougier P, Milan C, Lazorthes F, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Fondation Française de Cancérologie Digestive. Br J Surg. 1995;82:1397–1400. - PubMed
-
- Giacchetti S, Perpoint B, Zidani R, et al. Phase III Multicenter Randomized Trial of Oxaliplatin Added to Chronomodulated Fluorouracil-Leucovorin as First-Line Treatment of Metastatic Colorectal Cancer. J Clin Oncol. 2000;18:136–147. - PubMed
-
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J Clin Oncol. 2000;18:2938–2947. - PubMed
-
- Saltz L, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

