Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/μ receptor agonists in the sciatic nerve ligation chronic pain model in mice
- PMID: 21859931
- PMCID: PMC3199991
- DOI: 10.1124/jpet.111.184663
Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/μ receptor agonists in the sciatic nerve ligation chronic pain model in mice
Abstract
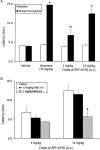
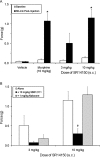
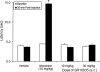
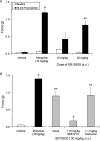
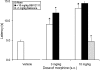
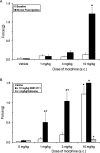
1-(1-Cyclooctylpiperidin-4-yl)-indolin-2-one (SR14150) and 1-(1-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)piperidinl-4-yl)-indolin-2-one (SR16835) are moderately selective nociceptin/orphanin FQ (NOP) receptor agonists. In the [(35)S]guanosine 5'-O-(3-thiotriphosphate) assay in vitro, SR14150 is a partial agonist at both the NOP and μ-opioid receptors, whereas SR16835 is a full agonist at the NOP receptor and has low efficacy at μ receptors. These compounds were tested for antinociceptive and antiallodynic activity, using mice in chronic pain, subsequent to spinal nerve ligation (SNL) surgery. When administered subcutaneously to mice after SNL surgery, SR14150 but not SR16835 increased tail-flick latency, which was blocked by the opioid antagonist naloxone, but not by the NOP receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). In contrast, both SR14150 and SR16835 had antiallodynic activity when mechanical allodynia was measured with von Frey monofilaments. This effect was completely blocked by SB-612111 but not by naloxone. On the other hand, morphine antinociception and antiallodynia were both blocked by naloxone and potentiated by SB-612111. These results indicate that, in mice, circuitry mediating antinociceptive activity in acute and chronic pain states is different. It is possible that during a chronic pain state, an up-regulated NOP system in the spinal cord leads to NOP receptor-mediated antiallodynia, which is blocked by NOP antagonists. However, supraspinal up-regulation could lead to an attenuation of morphine antinociception and antiallodynia, which can be alleviated by an NOP receptor antagonist. Thus, although neither NOP agonists nor antagonists are effective as analgesics in acute pain, they may have efficacy as analgesics, either alone or in combination with morphine, for treatment of chronic pain.
Figures
References
-
- Barlocco D, Cignarella G, Giuseppe G, Grugni M, Ronzoni S. (2006) inventors; GlaxoSmithKline S.p.A., assignee. Benzosuberonylpiperidine compounds as analgesics. U.S. patent 7,115,633 2006 Oct 3
-
- Bertorelli R, Bastia E, Citterio F, Corradini L, Forlani A, Ongini E. (2002) Lack of the nociceptin receptor does not affect acute or chronic nociception in mice. Peptides 23:1589–1596 - PubMed
-
- Briscini L, Corradini L, Ongini E, Bertorelli R. (2002) Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 447:59–65 - PubMed
-
- Carrà G, Rizzi A, Guerrini R, Barnes TA, McDonald J, Hebbes CP, Mela F, Kenigs VA, Marzola G, Rizzi D, et al. (2005) [(pF)Phe4,Arg14,Lys15]N/OFQ-NH2 (UFP-102), a highly potent and selective agonist of the nociceptin/orphanin FQ receptor. J Pharmacol Exp Ther 312:1114–1123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

