Comparison of azithromycin pharmacokinetics following single oral doses of extended-release and immediate-release formulations in children with acute otitis media
- PMID: 21859932
- PMCID: PMC3195035
- DOI: 10.1128/AAC.00692-11
Comparison of azithromycin pharmacokinetics following single oral doses of extended-release and immediate-release formulations in children with acute otitis media
Abstract
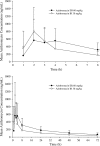
An azithromycin extended-release (ER) oral suspension was developed to improve the gastrointestinal tolerability profile without substantially compromising systemic exposure. A single dose of 30 mg/kg azithromycin immediate-release (IR) oral suspension has been used in children to treat acute otitis media (AOM). This study was conducted to compare the pharmacokinetics of a 60-mg/kg azithromycin ER single dose with a 30-mg/kg azithromycin IR single dose in children with AOM aged 6 months to 6 years (n = 19 per treatment). Serum samples were collected at 1, 2, 3, 4, 8, 24, 48, and 72 h after dosing. The area under the curve from time zero to 72 h postdosing (AUC(0-72)) was calculated based on a noncompartmental method. One-way analysis of variance (ANOVA) was used to compare exposure parameters (e.g., AUC(0-72) and peak concentration) as well as concentrations at each time point. The adjusted geometric mean ratio of the ER/IR AUC(0-72) was 157.98% (90% confidence interval [CI], 98.87%, 252.44%), which met the predefined criterion of the lower boundary of the 90% CI of ≥ 80%. As expected, due to the slower-release profile of the ER formulation, the concentrations of the ER formulation during the first 3 h were lower than those of the IR formulation. After 3 h postdosing, the lower boundaries of the 90% CI for the ER/IR concentration ratios were greater than 100%. These results indicated that a 60-mg/kg single dose of ER azithromycin provides similar or greater systemic exposure in children than the 30-mg/kg single dose of IR azithromycin.
Figures
References
-
- Andes D. 2001. Pharmacokinetic and pharmacodynamic properties of antimicrobials in the therapy of respiratory tract infections. Curr. Opin. Infect. Dis. 14:165–172 - PubMed
-
- Arguedas A., et al. 2005. A randomized, multicenter, double blind, double dummy trial of single dose azithromycin versus high dose amoxicillin for treatment of uncomplicated acute otitis media. Pediatr. Infect. Dis. J. 24:153–161 - PubMed
-
- Arguedas A., Loaiza C., Ferris J., Jorgensen D. 2007. Tolerability of extended-release azithromycin pediatric suspension (PEDS-AZM) vs. extended-release azithromycin adult suspension (ADULT-AZM) in children with acute otitis media (AOM), abstr. G-981. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007
-
- Arguedas A., Soley C., Jorgensen D. 2006. Single, high-dose azithromycin extended release (60 mg/kg) (AZ-ER) vs. 10 days high-dose amoxicillin clavulanate (AC) in children with acute otitis media (AOM) at high risk of persistent/recurrent otitis media (HR-P/ROM), abstr. G-347a. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006
-
- Chandra R., et al. 2007. Clinical pharmacokinetics and gastrointestinal tolerability of a novel extended-release microsphere formulation of azithromycin. Clin. Pharmacokinet. 46:247–259 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous