Cethromycin-mediated protection against the plague pathogen Yersinia pestis in a rat model of infection and comparison with levofloxacin
- PMID: 21859946
- PMCID: PMC3195012
- DOI: 10.1128/AAC.00632-11
Cethromycin-mediated protection against the plague pathogen Yersinia pestis in a rat model of infection and comparison with levofloxacin
Abstract
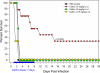
The Gram-negative plague bacterium, Yersinia pestis, has historically been regarded as one of the deadliest pathogens known to mankind, having caused three major pandemics. After being transmitted by the bite of an infected flea arthropod vector, Y. pestis can cause three forms of human plague: bubonic, septicemic, and pneumonic, with the latter two having very high mortality rates. With increased threats of bioterrorism, it is likely that a multidrug-resistant Y. pestis strain would be employed, and, as such, conventional antibiotics typically used to treat Y. pestis (e.g., streptomycin, tetracycline, and gentamicin) would be ineffective. In this study, cethromycin (a ketolide antibiotic which inhibits bacterial protein synthesis and is currently in clinical trials for respiratory tract infections) was evaluated for antiplague activity in a rat model of pneumonic infection and compared with levofloxacin, which operates via inhibition of bacterial topoisomerase and DNA gyrase. Following a respiratory challenge of 24 to 30 times the 50% lethal dose of the highly virulent Y. pestis CO92 strain, 70 mg of cethromycin per kg of body weight (orally administered twice daily 24 h postinfection for a period of 7 days) provided complete protection to animals against mortality without any toxic effects. Further, no detectable plague bacilli were cultured from infected animals' blood and spleens following cethromycin treatment. The antibiotic was most effective when administered to rats 24 h postinfection, as the animals succumbed to infection if treatment was further delayed. All cethromycin-treated survivors tolerated 2 subsequent exposures to even higher lethal Y. pestis doses without further antibiotic treatment, which was related, in part, to the development of specific antibodies to the capsular and low-calcium-response V antigens of Y. pestis. These data demonstrate that cethromycin is a potent antiplague drug that can be used to treat pneumonic plague.
Figures






References
-
- Agar S. L., et al. 2009. Characterization of the rat pneumonic plague model: infection kinetics following aerosolization of Yersinia pestis CO92. Microbes Infect. 11:205–214 - PubMed
-
- Alibek K., Handelman S. 1999. Biohazard: the chilling true story of the largest covert biological weapons program in the world - told from inside by the man who ran it. Random House Inc., New York, NY
-
- Alvarez M. L., Cardineau G. A. 2010. Prevention of bubonic and pneumonic plague using plant-derived vaccines. Biotechnol. Adv. 28:184–196 - PubMed
-
- Chen T. H., Foster L. E., Meyer K. F. 1974. Comparison of the immune response to three different Yersinia pestis vaccines in guinea pigs and langurs. J. Infect. Dis. 129(Suppl.):S53–S61 - PubMed
-
- Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—8th edition. M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

