Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses
- PMID: 21859995
- PMCID: PMC3188282
- DOI: 10.1200/JCO.2011.36.4539
Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses
Abstract
Purpose: The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial demonstrated that the addition of oxaliplatin to fluorouracil plus leucovorin (FULV) improved disease-free survival (DFS) in patients with stage II or III colon cancer. This analysis is the first publication of overall survival (OS) for the NSABP C-07 study. We updated DFS and examined both end points in clinically relevant patient subsets.
Patients and methods: Other studies have identified patients age 70 or older and those with stage II disease as patient subsets in which oxaliplatin may not be effective. We investigated toxicity as a driver of divergent outcomes in these subsets.
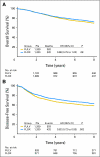
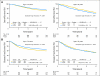
Results: In all, 2,409 eligible patients with follow-up were randomly assigned to either FULV (FU 500 mg/m(2) by intravenous [IV] bolus weekly for 6 weeks; leucovorin 500 mg/m(2) IV weekly for 6 weeks of each 8-week cycle for three cycles) or FLOX (FULV plus oxaliplatin 85 mg/m(2) IV on days 1, 15, and 29 of each cycle). With 8 years median follow-up, OS was similar between treatment groups (hazard ratio [HR], 0.88; 95% CI, 0.75 to 1.02; P = .08). FLOX remained superior for DFS (HR, 0.82; 95% CI, 0.72 to 0.93; P = .002). The effect of oxaliplatin on OS did not differ by stage of disease (interaction P = .38 for OS; interaction P = 0.37 for DFS) but did vary by age for OS (younger than age 70 v 70+ interaction P = .039). There was a similar trend for DFS (interaction P = .073). Oxaliplatin significantly improved OS in patients younger than age 70 (HR, 0.80; 95% CI, 0.68 to 0.95; P = .013), but no positive effect was evident in older patients.
Conclusion: Overall, the addition of oxaliplatin to FULV has not been proven to extend OS in this trial, but the DFS effect remained strong. Unplanned subset analyses suggest a significant OS effect of oxaliplatin in patients younger than age 70.
Trial registration: ClinicalTrials.gov NCT00004931.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med . 2004;350:2343–2351. - PubMed
-
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol . 2007;25:2198–2204. - PubMed
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC Trial. J Clin Oncol . 2009;27:3109–3116. - PubMed
-
- Schoenfeld D. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika . 1981;68:316–319.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

