TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms
- PMID: 21860020
- PMCID: PMC3236121
- DOI: 10.1182/blood-2011-04-348144
TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms
Abstract
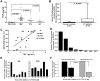
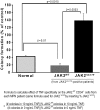
Proinflammatory cytokines such as TNFα are elevated in patients with myeloproliferative neoplasms (MPN), but their contribution to disease pathogenesis is unknown. Here we reveal a central role for TNFα in promoting clonal dominance of JAK2(V617F) expressing cells in MPN. We show that JAK2(V617F) kinase regulates TNFα expression in cell lines and primary MPN cells and TNFα expression is correlated with JAK2(V617F) allele burden. In clonogenic assays, normal controls show reduced colony formation in the presence of TNFα while colony formation by JAK2(V617F)-positive progenitor cells is resistant or stimulated by exposure to TNFα. Ectopic JAK2(V617F) expression confers TNFα resistance to normal murine progenitor cells and overcomes inherent TNFα hypersensitivity of Fanconi anemia complementation group C deficient progenitors. Lastly, absence of TNFα limits clonal expansion and attenuates disease in a murine model of JAK2(V617F)-positive MPN. Altogether our data are consistent with a model where JAK2(V617F) promotes clonal selection by conferring TNFα resistance to a preneoplastic TNFα sensitive cell, while simultaneously generating a TNFα-rich environment. Mutations that confer resistance to environmental stem cell stressors are a recognized mechanism of clonal selection and leukemogenesis in bone marrow failure syndromes and our data suggest that this mechanism is also critical to clonal selection in MPN.
Figures
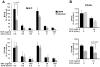

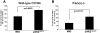
Comment in
-
Getting to know JAK.Blood. 2011 Dec 8;118(24):6235-7. doi: 10.1182/blood-2011-09-377291. Blood. 2011. PMID: 22161849 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

