Therapeutic potential and anti-amyloidosis mechanisms of tert-butylhydroquinone for Alzheimer's disease
- PMID: 21860091
- PMCID: PMC3686822
- DOI: 10.3233/JAD-2011-110512
Therapeutic potential and anti-amyloidosis mechanisms of tert-butylhydroquinone for Alzheimer's disease
Abstract
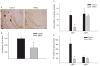
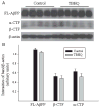
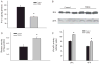
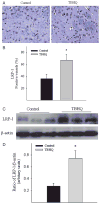
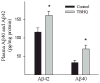
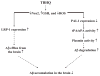
Alzheimer's disease (AD) is a major cause of dementia in the elderly with no effective treatment. Accumulation of amyloid-β peptide (Aβ) in the brain, one of the pathological features of AD, is considered to be a central disease-causing and disease-promoting event in AD. In this study, we showed that feeding male AβPP/PS1 transgenic mice, a well established mouse model of AD, with a diet containing phenolic antioxidant tert-butylhydroquinone (TBHQ) dramatically reduced brain Aβ load with no significant effect on the amounts of alpha- and beta-C-terminal fragments or full-length AβPP. Further studies showed that TBHQ diet inhibited the expression of plasminogen activator inhibitor-1 (PAI-1), a protease inhibitor which plays a critical role in brain Aβ accumulation in AD, accompanied by increases in the activities of tissue type and urokinase type plasminogen activators (tPA and uPA) as well as plasmin. Moreover, we showed that TBHQ diet increased the expression of low density lipoprotein related protein-1, a multi ligand endocytotic receptor involved in transporting Aβ out of the brain, and plasma Aβ(40) and Aβ(42) levels. We also showed that TBHQ diet increased the concentration of glutathione, an important antioxidant, and suppressed the expression of NADPH oxidase 2 as well as lipid peroxidation. Collectively, our data suggest that TBHQ may have therapeutic potential for AD by increasing brain antioxidant capacity/reducing oxidative stress level and by stimulating Aβ degradation/clearance pathways.
Figures

References
-
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A[beta] peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. - PubMed
-
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJF, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against [beta]-Amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. - PubMed
-
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. - PMC - PubMed
-
- Fukumoto H, Takahashi H, Tarui N, Matsui J, Tomita T, Hirode M, Sagayama M, Maeda R, Kawamoto M, Hirai K, Terauchi J, Sakura Y, Kakihana M, Kato K, Iwatsubo T, Miyamoto M. A noncompetitive BACE1 inhibitor TAK-070 ameliorates Aβ pathology and behavioral deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2010;30:11157–11166. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

