Self-organization phenomena in embryonic stem cell-derived embryoid bodies: axis formation and breaking of symmetry during cardiomyogenesis
- PMID: 21860211
- PMCID: PMC7615842
- DOI: 10.1159/000328712
Self-organization phenomena in embryonic stem cell-derived embryoid bodies: axis formation and breaking of symmetry during cardiomyogenesis
Abstract
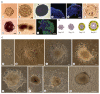
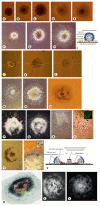
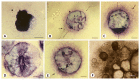
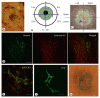
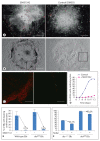
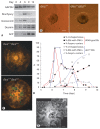
Aggregation of embryonic stem cells gives rise to embryoid bodies (EBs) which undergo developmental processes reminiscent of early eutherian embryonic development. Development of the three germ layers suggests that gastrulation takes place. In vivo, gastrulation is a highly ordered process but in EBs only few data support the hypothesis that self-organization of differentiating cells leads to morphology, reminiscent of the early gastrula. Here we demonstrate that a timely implantation-like process is a prerequisite for the breaking of the radial symmetry of suspended EBs. Attached to a surface, EBs develop a bilateral symmetry and presumptive mesodermal cells emerge between the center of the EBs and a horseshoe-shaped ridge of cells. The development of an epithelial sheet of cells on one side of the EBs allows us to define an 'anterior' and a 'posterior' end of the EBs. In the mesodermal area, first cardiomyocytes (CMCs) develop mainly next to this epithelial sheet of cells. Development of twice as many CMCs at the 'left' side of the EBs breaks the bilateral symmetry and suggests that cardiomyogenesis reflects a local or temporal asymmetry in EBs. The asymmetric appearance of CMCs but not the development of mesoderm can be disturbed by ectopic expression of the muscle-specific protein Desmin. Later, the bilateral morphology becomes blurred by an apparently chaotic differentiation of many cell types. The absence of comparable structures in aggregates of cardiovascular progenitor cells isolated from the heart demonstrates that the self-organization of cells during a gastrulation-like process is a unique feature of embryonic stem cells.
Copyright © 2011 S. Karger AG, Basel.
Figures
Similar articles
-
Assessment of differentiation aspects by the morphological classification of embryoid bodies derived from human embryonic stem cells.Stem Cells Dev. 2011 Nov;20(11):1925-35. doi: 10.1089/scd.2010.0476. Epub 2011 Apr 19. Stem Cells Dev. 2011. PMID: 21388292
-
Serum withdrawal after embryoid body formation does not impair cardiomyocyte development from mouse embryonic stem cells.Cytotherapy. 2011 Mar;13(3):350-6. doi: 10.3109/14653249.2010.520311. Epub 2010 Sep 27. Cytotherapy. 2011. PMID: 20873992
-
Cardiogenesis of embryonic stem cells with liquid marble micro-bioreactor.Adv Healthc Mater. 2015 Jan 7;4(1):77-86. doi: 10.1002/adhm.201400138. Epub 2014 May 12. Adv Healthc Mater. 2015. PMID: 24818841
-
The role of Hippo signaling pathway and mechanotransduction in tuning embryoid body formation and differentiation.J Cell Physiol. 2020 Jun;235(6):5072-5083. doi: 10.1002/jcp.29455. Epub 2020 Jan 17. J Cell Physiol. 2020. PMID: 31951024 Review.
-
Cell Mechanics in Embryoid Bodies.Cells. 2020 Oct 11;9(10):2270. doi: 10.3390/cells9102270. Cells. 2020. PMID: 33050550 Free PMC article. Review.
Cited by
-
Deconstructing and reconstructing the mouse and human early embryo.Nat Cell Biol. 2018 Aug;20(8):878-887. doi: 10.1038/s41556-018-0144-x. Epub 2018 Jul 23. Nat Cell Biol. 2018. PMID: 30038253 Review.
-
Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures.Cell. 2019 Oct 17;179(3):687-702.e18. doi: 10.1016/j.cell.2019.09.029. Cell. 2019. PMID: 31626770 Free PMC article.
-
The Trophoblast Compartment Helps Maintain Embryonic Pluripotency and Delays Differentiation towards Cardiomyocytes.Int J Mol Sci. 2023 Aug 4;24(15):12423. doi: 10.3390/ijms241512423. Int J Mol Sci. 2023. PMID: 37569800 Free PMC article.
-
High-resolution transcriptional and morphogenetic profiling of cells from micropatterned human ESC gastruloid cultures.Elife. 2020 Nov 18;9:e59445. doi: 10.7554/eLife.59445. Elife. 2020. PMID: 33206048 Free PMC article.
-
Genetically-stable engineered optogenetic gene switches modulate spatial cell morphogenesis in two- and three-dimensional tissue cultures.Nat Commun. 2024 Dec 2;15(1):10470. doi: 10.1038/s41467-024-54350-7. Nat Commun. 2024. PMID: 39622829 Free PMC article.
References
-
- Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, Villageois P, Barhanin B, Vernallis A, Smith AG, Ailhaud G, et al. Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem. 1999;274:24965–24972. - PubMed
-
- Bader A, Al-Dubai H, Weitzer G. Leukemia inhibitory factor modulates cardiogenesis in embryoid bodies in opposite fashions. Circ Res. 2000a;86:787–794. - PubMed
-
- Bader A, Gruss A, Höllrigl A, Al-Dubai H, Capetanaki Y, Weitzer G. Paracrine promotion of cardiomyogenesis in embryoid bodies by LIF modulated endoderm. Differentiation. 2001;68:31–43. - PubMed
-
- Bader M, Bohnemeier H, Zollmann FS, Lockley-Jones OE, Ganten D. Transgenic animals in cardiovascular disease research. Exp Physiol. 2000b;85:713–731. - PubMed
-
- Behr R, Heneweer C, Viebahn C, Denker HW, Thie M. Epithelial-mesenchymal transition in colonies of rhesus monkey embryonic stem cells: a model for processes involved in gastrulation. Stem Cells. 2005;23:805–816. - PubMed

