Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis
- PMID: 21860392
- PMCID: PMC3280198
- DOI: 10.1038/nrm3175
Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis
Abstract
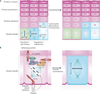
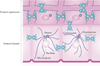
To provide a stable environmental barrier, the epidermis requires an integrated network of cytoskeletal elements and cellular junctions. Nevertheless, the epidermis ranks among the body's most dynamic tissues, continually regenerating itself and responding to cutaneous insults. As keratinocytes journey from the basal compartment towards the cornified layers, they completely reorganize their adhesive junctions and cytoskeleton. These architectural components are more than just rivets and scaffolds - they are active participants in epidermal morphogenesis that regulate epidermal polarization, signalling and barrier formation.
Figures



References
-
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nature Rev. Genet. 2002;3:199–209. - PubMed
-
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature Rev. Mol. Cell Biol. 2005;6:328–340. - PubMed
-
- Mack JA, Anand S, Maytin EV. Proliferation and cornification during development of the mammalian epidermis. Birth Defects Res. C Embryo Today. 2005;75:314–329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

