Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer
- PMID: 21860420
- PMCID: PMC3397306
- DOI: 10.1038/onc.2011.350
Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer
Abstract
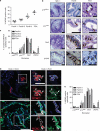
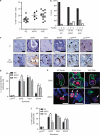
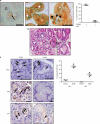
During tumor initiation, oncogene-induced senescence (OIS) is proposed to limit the progression of preneoplasms to invasive carcinoma unless circumvented by the acquisition of certain tumor suppressor mutations. Using a variety of biomarkers, OIS has been previously reported in a wide range of human and murine precursor lesions, including the pancreas, lung, colon and skin. Here, we have characterized a panel of potential OIS biomarkers in human and murine pancreatic intraepithelial neoplasia (PanIN), and found that only senescence-associated β-galactosidase (SAβgal) activity is specifically enriched in these precursors, compared with pancreatic ductal adenocarcinoma (PDA). Indeed, many of the other proposed OIS biomarkers are detected in actively proliferating PanIN epithelium and in cells within the microenvironment. Surprisingly, acinar to ductal metaplasia (ADM), a distinct preneoplasm that is potentially a precursor for PanIN, also exhibits SAβgal activity and contains a higher content of p21 and p53 than PanIN. Therefore, SAβgal activity is the only biomarker that accurately identifies a small and heterogeneous population of non-proliferating premalignant cells in the pancreas, and the concomitant expression of p53 and p21 in ADM supports the possibility that PanIN and ADM each exhibit discrete senescence blocks.
Figures

References
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. - PubMed
-
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
-
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

